A Guide to Clear-Cut Planning and Program Execution
The landscape of clinical trials and other clinical development projects has dramatically changed in recent years. Progress made in scientific discovery is paving the way to novel therapies and...
Do You Need a Project Management Office (PMO)?
There is more to a project management office than managing projects. In fact, the project management office (PMO) acts as both a watchtower and a lighthouse to guide strategic initiatives toward...
7 Medical Writing Tips for the Oncology Field
In the wake of the 21st Century Cures Act (December 2016), the FDA has switched gears to accelerate the development of novel therapies and speed up the review process, particularly in the field of...

Medical Writing Lean Overview
When applied rigorously and comprehensively, lean principles can reduce organizational costs while increasing productivity and strengthening the overall quality of submissions What is “Lean” as it...
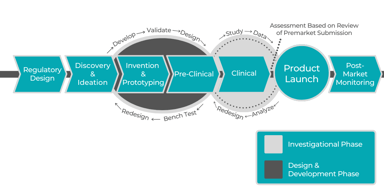
Pharmaceutical Consulting during Product & Business Development
Pharmaceutical Consulting during Product & Business Development: To stay competitive in the pharmaceutical field, pharmaceutical companies need to have access to legal, product and clinical...
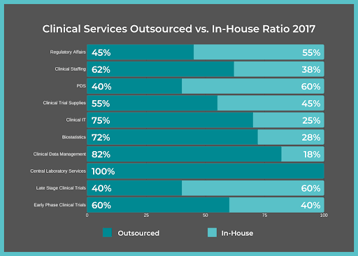
5 Ways a Functional Service Provider Can Support Your Clinical Development Project
When pharmaceutical companies launch a clinical trial or reach a certain phase of Clinical Development, with only the support of their in-house employees, the additional workload often becomes too...

6 Compliance Tips to get FDA Approval for Your Pharmaceutical Project
FDA Approval Process Overview The Food and Drug Administration (FDA), as part of the United States (US) Department of Health and Human Services, is the regulatory agency responsible for the review,...





