Clinical Research Solutions
Do You Need a Project Management Office (PMO)?
There is more to a project management office than managing projects. In fact, the project management office (PMO) acts as both a watchtower and a lighthouse to guide strategic initiatives toward...
Clinical Research Solutions
7 Medical Writing Tips for the Oncology Field
In the wake of the 21st Century Cures Act (December 2016), the FDA has switched gears to accelerate the development of novel therapies and speed up the review process, particularly in the field of...
Clinical Research Solutions
Changes in Medical Device Regulatory Requirements in Europe (2021)
In May 2021, the new European regulations on medical devices (EU MDR) will take full effect. Organizations face a unique set of compliance challenges, mainly due to the numerous changes and additions...
Clinical Research Solutions
FDA: CGMP Consultant Recommendation
Reflecting on my interactions with FDA I have come to understand that inspectors do not routinely offer recommendations to firms, when an inspector does provide an opinion it is often prefaced with a...
Meet the Expert: Dr. Borja López Pérez
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to support...

Clinical Research Solutions
The Importance of the eCTD Structure for FDA Approval
The Importance of the eCTD To harmonize the process of regulatory reviews for global drug development, a unified structure was developed and implemented for electronic Common Technical Document...
Clinical Research Solutions
Establishing Process Equivalency
A common question when transferring an established process from one facility to another is how to establish that the transferred process is performing equivalently to the original process. Sounds...

Clinical Research Solutions
Medical Writing Lean Overview
When applied rigorously and comprehensively, lean principles can reduce organizational costs while increasing productivity and strengthening the overall quality of submissions What is “Lean” as it...

Clinical Research Solutions
6 Unique Challenges Hindering Oncology Clinical Trials
The research for cancer treatment is moving forward at a rapid pace. We're witnessing a shift from chemotherapy protocols to MTAs (molecularly targeted agents) for immunotherapies in particular....
Clinical Research Solutions
How to Safely Launch Medical Cannabis Products in Germany
Over the past few weeks the withdrawal of cannabis products from the German market has been a topic which generated a lot of publicity. Therefore, we want to share some tips on how to safely launch...
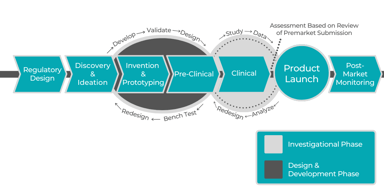
Clinical Research Solutions
Pharmaceutical Consulting during Product & Business Development
To stay competitive in the pharmaceutical field, pharmaceutical companies need to have access to legal, product and clinical development, business strategy, and human resources experts. Startups...
Regulatory Sciences
Three Steps Towards Complying with Nitrosamine Regulations
All Marketing Authorization Holders (MAH) of medicines for human use are facing what might feel for many, as a new requirement: review their drug products on the possible presence of nitrosamines....
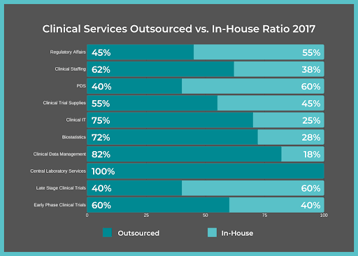
FSP Solutions
5 Ways a Functional Service Provider Can Support Your Clinical Development Project
When pharmaceutical companies launch a clinical trial or reach a certain phase of Clinical Development, with only the support of their in-house employees, the additional workload often becomes too...
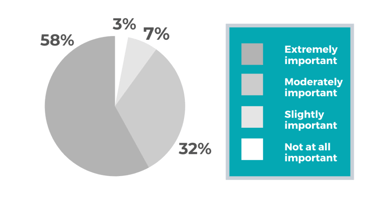
Clinical Research Solutions
Life Sciences Data Analytics: The Importance of Big Data
Big data is hitting us from all angles, and life science industries are not being left out. Why? Your life depends on it, literally. Life sciences generate lots of large and complex data every single...
Clinical Research Solutions
6 Compliance Tips to get FDA Approval for Your Pharmaceutical Project
FDA Approval Process Overview The Food and Drug Administration (FDA), as part of the United States (US) Department of Health and Human Services, is the regulatory agency responsible for the review,...
Clinical Research Solutions
Gene and Cellular Therapies: Five Keys to Regulatory Success
We are living through a medical revolution. Advances in gene therapy, cell‑based therapies and tissue engineering offer real hope for patients with a range of debilitating diseases. The FDA, EMA and...