
Deviations: Beyond the Basics
There are plenty of guidelines and instructions on implementing a deviation system in a pharmaceutical/medical device company. However, there is a big difference between theory and practice when it...

Why the FDA Should Never Be Your First Inspection
You can expect several FDA audits throughout your drug development program The Agency’s goal is to protect the public from unsafe products, and one of the best ways to accomplish that goal is by...
Clinical Research Solutions
FDA's Top 483 Observations for 2018: A Reflection of Industry’s Compliance
At the beginning of each federal fiscal year, the US FDA posts the previous year's Form 483 observation metrics issued by each product center. Inspections ending between 10/1/2017 and 9/30/2018, for...
Meet the Expert: Mary Speckin
ProPharma Group has launched a "Meet the Expert" series to introduce you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to support...
Clinical Research Solutions
Assumed Brexit & Batch Control Testing Site In the UK
The currently scheduled transition date in the Brexit process, 30 March 2019, is coming very close. In light of this, the European Commission (EC) published on 25 February 2019 a notice on the...

Clinical Research Solutions
Year in Review: Taking a Look at Pharma's Top News Stories & Events from 2018
From FDA's approval of the first cannabis-based product in the U.S., to the classification of two Apple Watch apps and changes in the Agency's submission requirements related to Sponsor meetings,...
Innocent Until Proven Guilty: Hypothesis Test
Each of us makes important decisions that shape our lives, every day. We try to make the best decisions possible and yet, as the adage goes "all predictions of the future are wrong, some are just...
Clinical Research Solutions
Outsourcing Functions Doesn't Mean You've Outsourced Compliance Obligations
Over the past 20 years, the traditional approach to drug development has expanded to include the outsourcing of a range of testing and manufacturing functions. As a part of their long-term strategic...

Clinical Research Solutions
Human Subject Protection Regulations: Differences Between HHS’ & FDA’s Clinical Trial Rules
On Friday, October 12th, FDA issued a guidance document entitled “Impact of Certain Provisions of the Revised Common Rule on FDA-Regulated Clinical Investigations.” The document aims to help...

Regulatory Sciences
CBER Provides Sponsors with Policies and Procedures Regarding INTERACT Meetings
On Monday, October 1, FDA’s Center for Biologics Evaluation and Research (CBER) issued a document outlining the policies and procedures for scheduling and conducting INitial Targeted Engagement for...

Good Review Management Principles & Practices, Part One: Fundamental Values
On Tuesday, September 25th, the FDA published a draft guidance containing recommendations on good review management principles and practices (GRMPs) for new drug applications (NDAs), Biologics...
Clinical Research Solutions
FDA Releases Draft Guidance on Benefit-Risk Determinations for Devices
On Thursday, September 6th, the FDA released a new draft guidance regarding benefit-risk determinations in medical device premarket approval applications (PMAs), De Novo requests, and humanitarian...
Regulatory Sciences
Generic Failure: Why so Few ANDAs Are Accepted by FDA on the First Pass
Generic drugs are immensely important to the U.S. healthcare system. These drugs account for 89% of the prescriptions dispensed in the United States. And, over the last decade, generic drugs have...
Clinical Research Solutions
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...
Clinical Research Solutions
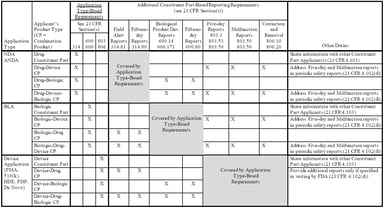
Understanding the New Combination Product PMSR Guidance Documents and Impact on Industry
On March 20, 2018, the US Food and Drug Administration (FDA) released two new guidance documents to help companies comply with the December 20, 2016 final rule establishing postmarketing safety...
Clinical Research Solutions
FDA Steps up its Game on Generic Drugs: The Story Behind the Recent Focus on Generic Products
Throughout 2017, the FDA focused its attention on the regulation of generic drug products. In 2015, the Agency issued only two generic-related guidance documents. In 2016, there were seven. In 2017,...