
Meet the Expert: James Meckstroth
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Roadmap for Successful IVDR Transition, Part III: Project Management
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching in May 2022. In this blog series, we discuss the final months before the IVDR date of application, how to...
Do You Know Your Quality Score?
We live in a world of measurement and metrics. Running a successful business requires a thorough analysis on the work, sales, and financial results. Of course, identifying and tracking business...

Meet the Expert: Mary Dederich
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Why You Need a Decentralized Clinical Trials Strategy - Before Disaster Strikes
Imagine having your clinical trial ready to go, or already underway, and overnight the brakes are slammed, bringing your trial to a screeching halt. This was the reality for sponsors around the world...
What the IVDR Is and How to Prepare
In May 2022, the IVDD will be repealed by the European Committee, thereby ending the transition period. To ensure you're compliant with IVDR by that date, learn everything you need to know about the...
Battle of the Regulators: Comparing FDA’s Accelerated Approval and EMA’s Conditional Marketing Authorisation
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) offer various pathways that can expedite the development and review of new therapies to treat serious or...
Quality & Compliance
Roadmap for Successful IVDR Transition, Part II: Technical Documentation & Software
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching (May 2022). In this blog series, we discuss the final months before the IVDR date of application along with...
CMC Regulatory Dossier Compliance: A GMP Requirement
Maintaining compliance in the dynamic regulatory Chemistry, Manufacturing and Controls (CMC field can be quite a challenge. A CMC regulatory dossier compliance assessment is a critical component and...
Meet the Expert: Marshall Scicchitano
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Regulatory Sciences
FDA’s Breakthrough Therapy Designation vs PRIority MEdicines (PRIME) Application in Europe
What are Breakthrough Therapy Designation and PRIority MEdicines (PRIME) Applications? The advancement of modern medicine, and the accessibility of researched and regulated medication, has greatly...
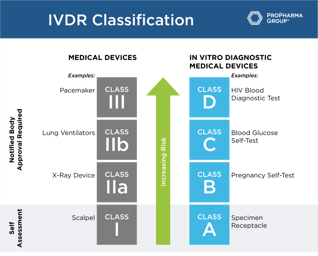
Quality & Compliance
Roadmap for Successful IVDR Transition
Roadmap for Successful IVDR Transition: The compliance dates for the In Vitro Diagnostics Regulation (IVDR) will become effective on May 26, 2022. To help you with the IVDD to IVDR transition, we've...
Quality & Compliance
Top Questions & Answers for Managing the New IVDR
With an increased need for high quality in vitro diagnostic medical devices (IVDs), the In Vitro Diagnostic Regulation (IVDR, 2017/746) was entered into force for all IVDs in 2017 with a five-year...
Should You Undergo Virtual Factory Acceptance Testing?
Although domestic travel is slowly ramping up, companies may still consider virtual factory acceptance testing (FAT). FAT is an optional step in the life sciences’ process of purchasing factory...
Clinical Research Solutions
Regulatory Strategy for Clinical Trials in the European Union
Setting up a clinical trial in the European Union (EU) has historically been an expensive and time-consuming business. With 27 individual member states each requiring its own review and approval, it...






