Regulatory Sciences
How to Present Scientific Data That Generates Regulatory Approvals
Today’s patients benefit from having access to the safest, most advanced pharmaceutical systems around the world. This is because of the work done by the various regulatory bodies who govern the...

Meet the Expert: Hanna Edling
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Have an FDA Submission on Your 2021 To-Do List?
At the start of every year, we all have these grand plans of everything we plan to accomplish. It is a fresh start to really get stuff done and we have a full 12 months to do it all. However,...

Meet the Expert: Bram Lardée
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
How to Implement an Effective Audit Trail
Maintaining an audit trail is a regulatory compliance requirement, but what makes an audit trail beneficial for maintaining effectiveness and complying with regulations? This blog will explain what...
Quality & Compliance
Prepare for Your Next Audit: A 5-Point GMP Checklist
Ensuring you have full control over your processes, facility, and quality management system (QMS), and ultimately your final product quality, is a demanding and important task. An inability to do so...
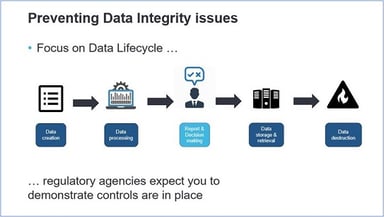
Measuring the Maturity of Data Integrity
We live in a world of data: there’s more of it than ever before, in a ceaselessly expanding array of forms and locations. Besides this, most people in their organizations are not always aware of data...
6 Benefits of Custom PMO Design
A Project Management Office ("PMO") can be incredibly advantageous to large companies with many ongoing projects across various job sites. A PMO streamlines project management processes to make a...

Meet the Expert: Marla Scarola
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Regulatory Sciences
Life Science Outsourcing: Not sure you’re ready to turn over the keys?
It's no secret. To be successful in the life sciences industry you must accomplish a lot – achieve and maintain profitable growth, deliver safe and effective products to patients quickly, manage...
Regulatory Sciences
Understanding The Medical Device Single Audit Program
The Medical Device Single Audit Program (MDSAP), initiated by the International Medical Device Regulators Forum (IMDRF), created a global approach to auditing and monitoring the manufacturing of...
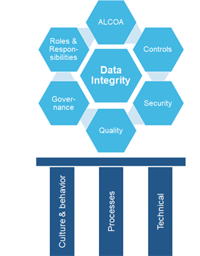
Creating Efficiencies with Data Integrity
Over recent years relations with data and its weight have changed significantly; consequently, an assessment of Company Data Integrity is becoming one of the key metrics of corporate functionality...
Regulatory Sciences
The Human Factor - Preparing Your Device for Usability Testing
When it comes to usability studies, the focus should be on effectively preparing the medical device for use in humans. Whether a Sponsor is conducting formative testing or validation testing, the...
Quality & Compliance
Innovation and Rapid Growth: A Double-Edged Sword
Every industry has a distinct set of obstacles to overcome, but it’s no secret that the life sciences industry encounters more speedbumps than most. Market cycles and product failures are expected,...

FDA Guidance on Gene Therapy Products: RCR Testing and Monitoring – Focus on Documenting Test Results & Post-Licensure Considerations
In January 2020, FDA issued a final guidance document on the topic of "Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture...






