Quality & Compliance
Post-COVID Challenges with Investigation Backlog: Are You Ready for the Inevitable?
In early 2020, while COVID-19 was wreaking havoc on public health and safety, the FDA took the unprecedented step of postponing domestic and foreign inspections. The FDA’s risk-based inspection...
Quality & Compliance
FDA Guidance on Gene Therapy Products: RCR Testing and Monitoring
Gene therapy holds the promise of curing severe genetic diseases at the genetic level rather than merely treating the symptoms as is accomplished using conventional small-molecule drug therapies. In...
How to Prepare for the Three Type B Meetings with the FDA
The Food and Drug Administration (FDA) has laid out a drug development continuum that includes three milestones, or Type B meetings. Earliest is the Pre-IND meeting, the second is the End of Phase 2...
Regulatory Sciences
How to Understand and Avoid Common Phase 3 Failure Points
By the time the drug development process gets to Phase 3, it seems reasonable to assume that the chance of failure is relatively low. After all, the entire early-development process is at least...
Regulatory Sciences
Meet the Expert: Carrie Rabe
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Quality & Compliance
What is Software Quality Assurance (SQA) and Do You Need it For Your Business?
If your only method of quality assurance is software testing, you're wasting valuable time and resources. Software Quality Assurance (SQA) is integrated into your software development life cycle to...
Quality & Compliance
Understanding Responsible Person and Wholesaler Dealers Authorization
Wholesaling is a key factor for drug manufactures and Marketing Authorization Holders (MAH) to ensure supply of pharmaceutical products to their customers at pharmacies and hospitals. Wholesaling of...
Quality & Compliance
Meet the Expert: Gary Hyde
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Regulatory Sciences
The Anniversary We Didn’t Want: One Year of COVID-19 Milestones
On March 11, 2020, after months of researching, strategizing, and meeting with various leaders and medical experts globally, the World Health Organization (WHO) declared COVID-19 to be a global...

Quality & Compliance
Project Management and a Successful Reduction in Investigations Backlog: The Beauty and the Beast
Many of us have been faced with this beast that needs taming: a regulatory agency has conducted an inspection of your facility. Their observation is that the backlog of investigations at your site is...
Understanding FDA Pre-ANDA Meetings
Brought into being by the Generic Drug User Fee Amendments Reauthorization of 2017 (GDUFA II), the FDA’s pre-ANDA program is designed to accelerate access to generic versions of complex products. The...
Quality & Compliance
5 Steps to CMO Identification and Selection for Cell and Gene Therapies
After assuring clinical validity, finding and managing the right contract manufacturing organizations (CMOs)/contract development manufacturing organizations (CDMOs) is a Sponsor's major concern when...
Regulatory Sciences
The Unique Challenges of Gaining Approval for Drug-Device Combos
A combination product is composed of any combination of a drug and a device; a biological product and a device, a drug and a biological product, or a drug, device, and a biological product. Consider...
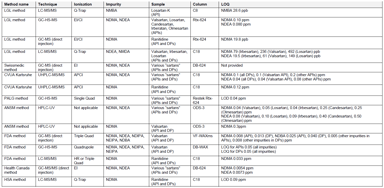
Analytical Methods for the Investigation of Carcinogenic Nitrosamines in APIs and Drug Products
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...
Clinical Research Solutions
The Quality of Your Medical Writing Matters (And Here’s Why)
Medical writers need to meet a few critical criteria before they publish their work. First and foremost, people want to see accuracy and explicit knowledge of the field when they’re seeking out...






