%20inspection%20procedures.jpeg?width=384&height=256&name=EMA%20Good%20clinical%20practice%20(GCP)%20inspection%20procedures.jpeg)
EMA Good clinical practice (GCP) inspection procedures
The Good Clinical Practice (GCP) Inspectors Working Group has developed procedures for the coordination, preparation, conduct and reporting of GCP inspections requested by the European Medicines...
Orphan Drug Designations in the US and EU
What is an Orphan Drug Designation? The Orphan Drug Designation (ODD) program in both the United States (U.S.) and European Union (E.U.) qualifies sponsors to receive potential incentives to develop...
Regulatory Sciences
Global regulators agree on key principles on adapting vaccines to tackle virus variants
On 30 June, regulators from around the world discussed emerging evidence to support adaptation of COVID-19 vaccines as the SARS-COV-2 virus continues to evolve during a workshop co-chaired by the...

Regulatory Sciences
Clinical Pharmacology Considerations for Oligonucleotides
For the first time, the FDA has issued a draft guidance for industry on “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics”. Oligonucleotides are short single...
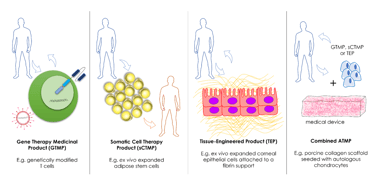
How to Leverage EMA’s ATMP Classification
Advanced therapy medicinal products (ATMPs) have emerged as ground-breaking therapies for rare diseases and other conditions with unmet clinical needs. As of 2022, sixteen ATMPs have been approved by...

Quality & Compliance
The 5 Phases of Project Management + Pro Tips for Success
Guilty as charged! I used to think a project manager’s (PM) sole job was to remind everyone about deadlines and set up status meetings. I learned firsthand while working at a medical device start-up,...

Meet the Expert: Jennifer Daudelin
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Project Management isn’t brain surgery, but why is it so hard?
Here is the scenario: you are the leader of a group and you have been given the responsibility of driving the completion of a new initiative, an early or late-stage drug or devices program, or a...
Maximising on Scientific Advice Procedures in Europe
A unique opportunity to interact with medicine regulators in Europe Are you considering requesting scientific advice in Europe? We can help you navigate the various procedures within the European...
EMA adopts first list of critical medicines for COVID-19
News On 7 June 2022, EMA's Medicines Shortages Steering Group (MSSG) adopted the list of critical medicines for the COVID-19 public health emergency. The medicines included in the list are authorised...

Meet the Expert: Elina Koli
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Clinical Research Solutions
What is Product Lifecycle Management and Why is it Critical for Success?
Product Lifecycle Management (PLM) is the process of managing a product from conception through end of of life (EOL), and clearly includes conception (design and development) and commercialization....

Understanding ICH M10 “Bioanalytical Method Validation and Study Sample Analysis"
The final version of ICH M10 “Bioanalytical Method Validation and Study Sample Analysis” was adopted on May 24, 2022. This is the harmonized guideline which has been ratified by participating...

FDA publishes MAPP 5223.6, Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA
Today, FDA published a new Manual of Policies and Procedures (MAPP), “Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA (5223.6).”...







