FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
Regulatory Sciences
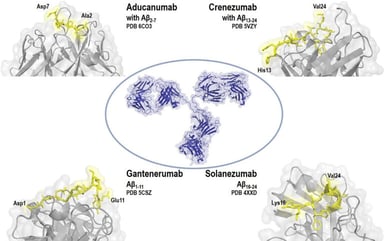
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Regulatory Sciences
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...
Regulatory Sciences
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...

Regulatory Sciences
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA), FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...

Clinical Research Solutions
From Idea to Market: The Five Stages of Product Development
The exact product development process for medical devices differs from region to region, with different regulatory expectations that need to be met in the EU, USA, UK and other regions. These precise...

Meet the Expert: Renata Pankiewicz
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Regulatory Sciences
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement
Pharmaceutical companies should understand EU Health Technology Assessment (HTA) authorities requirements early in the product development phase. Engagement with HTA authorities during clinical...
Regulatory Sciences
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

Regulatory Sciences
FDA Recognizes August as National Immunization Awareness Month
National Immunization Awareness Month provides us an opportunity to think about how far the development and advancement of immunization science has come, and its impact on public health. The U.S....

Regulatory Sciences
FDA publishes product-specific guidances to facilitate generic drug development
Today, FDA published a new batch of product-specific guidances (PSGs). PSGs provide recommendations for developing generic drugs and generating the evidence needed to support abbreviated new drug...

Regulatory Sciences
UK Paediatric Investigational Plans – what do you need to know?? …and how is it all working in practice??
If a marketing authorisation is planned to be submitted in England, Scotland, and Wales (GB), an MHRA-approved paediatric investigational plan (PIP) is required. Up until January 1, 2021, PIPs were...

EMA: Big data use for public health: publication of Big Data Steering Group workplan 2022-25
News July 28, 2022 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its third workplan that sets key actions to be delivered between 2022–25. The new...
How to Fast-Track medicine approval in the UK with the MHRA’s Innovative Licensing and Access Pathway (ILAP)
What is ILAP? What benefits does ILAP provide? How do you access it? With the dust of Brexit settling, the question on most people’s lips (well, those of us in the healthcare sector anyway!) was:...







