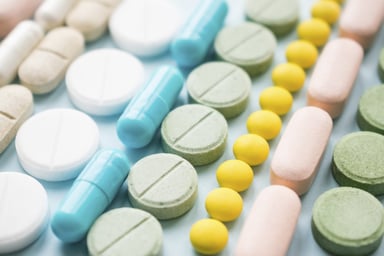
Ban on Titanium Dioxide (E171) on the EU Food Market: What Are the Consequences for Medicines?
Use of Titanium Dioxide in the EU Food Market In 2021, the European Food Safety Authority (EFSA) investigated the safety of the white coloring agent titanium dioxide (TiO2) and concluded that the...

Be Careful What You Ask For (Prior to Consent)
According to FDA's clinical trial regulations (21 CFR 50.20, 312.60 and 812.100), clinical investigators are responsible for protecting the rights, safety, and welfare of subjects during a clinical...

Meet the Expert: Sharon Charles
Our "Meet the Expert" series introduces you to our team of experts around the world. This "behind the curtain" view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: Jennifer Daudelin
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: Elina Koli
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Clinical Research Solutions
FDA Releases Draft Guidance for Industry on Clinical Pharmacology Considerations for Human Radiolabeled Mass Balance Studies
The FDA’s Office of Clinical Pharmacology within the Office of Translational Sciences released a new draft guidance document that, for the first time, clearly addresses the FDA’s recommendations and...

Meet the Expert: Steven Silverman
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Autism Awareness: Advancing Early Diagnosis in Children
Did you know that 1 in 44 American children is autistic, and approximately 5.4 million adults in the U.S. are autistic as well? It's an extraordinarily diverse group too, as autism spectrum disorder...

Meet the Expert: Jeff Block
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: Raphael Richarz
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: Shelby Stillwagon
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: James Meckstroth
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Do You Know Your Quality Score?
We live in a world of measurement and metrics. Running a successful business requires a thorough analysis on the work, sales, and financial results. Of course, identifying and tracking business...

Meet the Expert: Mary Dederich
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Battle of the Regulators: Comparing FDA’s Accelerated Approval and EMA’s Conditional Marketing Authorisation
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) offer various pathways that can expedite the development and review of new therapies to treat serious or...
Meet the Expert: Marshall Scicchitano
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...