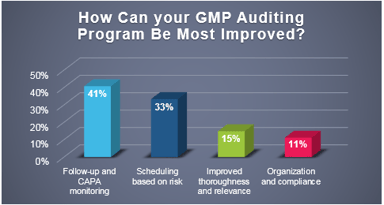
Industry Poll: How Can Your GMP Auditing Program Be Most Improved
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...
The Important Role of Advanced Therapy Medicinal Products
Increasing safe and effective patient treatment opportunities for the future is one of the driving forces behind ProPharma Group’s business. It’s also the driving force behind why many individuals...
Outdated Facilities – Bring Back the ‘c’ in cGMP
New rules, old facilities. How do these two meet? Is it a big black hole or is there light at the end of the tunnel? When you work in an older facility, you are probably acquainted with one-liners...
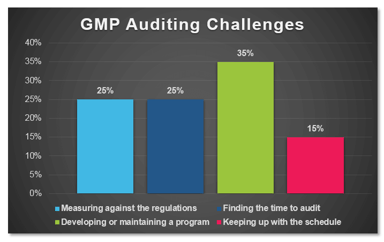
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
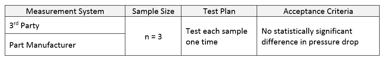
The Power to See Differences that Matter
Data collection and analysis is expensive and has the potential to compromise a company’s hard-won compliance position. It is therefore critical that technical leaders follow a systematic and proven...
MassBio Annual Meeting: Event Highlights
MassBio is a non-profit organization that represents and provides support for the life sciences supercluster in Massachusetts. MassBio is committed to growing the industry, adding value to...
Clinical Research Solutions
Transformational Leadership: Part I
A couple of years ago, an organization asked me to present to their Leadership Team regarding transformational leadership. They wanted to have a better understanding of what makes an organization...
Phase-Appropriate Development and Application of Quality Systems in the Drug Development Process
Phase-Appropriate Development and Application of Quality Systems in the Drug Development Process: The Parenteral Drug Association (PDA) recently published a revised version of Technical Report No....
Clinical Research Solutions
Understanding the 21st Century Cures Act: Part II
The 21st Century Cures Act, (Passed December 13, 2016), represents three years of cooperation between Congress, the FDA, and industry to modernize the current legal framework within which the FDA...
PDA Outsourcing / CMO Conference: A Review by Bob Beall
On November 3rd and 4th, I had the opportunity to meet with Pharmaceutical industry experts at the PDA Outsourcing / CMO conference in Washington, DC. It was a fantastic event with representatives...
Clinical Research Solutions
cGMP for Phase 1 Investigational Drugs: Sterile Parenteral Drug Product Manufacturing
As Phase I clinical trials mark the first time that an investigational new drug is administered to humans, these studies are subject to appropriate current Good Manufacturing Practices (cGMP) in...
Gravity and Quality Assurance
As we’ve now moved on from another cold winter month, I was captivated recently thinking about Quality Assurance and our collective responsibilities. All kinds of things come to mind; Deviations,...

Are You Maximizing Your Return on Data Investment?
Generating process data is expensive: Costs include materials, process time and the focus of highly-compensated subject matter experts. Unfortunately, many organizations don’t get their money’s worth...
Are You Ready for AIQ?
Do you find yourself in one of the following situations? + Your equipment has broken down due to frequent usage or wear and thus requires repair. You realize that once repaired, any equipment will...
Looking to the Cloud for your Business
computer susteIt’s a Friday afternoon. Quarter’s end. Your V.P. of Regulatory Affairs calls your office bellowing something about not being able to process the latest submission data- can’t access...
Clinical Research Solutions
If You Didn't Write It Down...
Since the day I entered the industry the mantra of "If you didn’t write it down, it didn’t happen" was repeatedly drilled into my daily mode of operation. The margins of my test sheets were filled...