
Quality & Compliance
Project Management and a Successful Reduction in Investigations Backlog: The Beauty and the Beast
Many of us have been faced with this beast that needs taming: a regulatory agency has conducted an inspection of your facility. Their observation is that the backlog of investigations at your site is...
Quality & Compliance
5 Steps to CMO Identification and Selection for Cell and Gene Therapies
After assuring clinical validity, finding and managing the right contract manufacturing organizations (CMOs)/contract development manufacturing organizations (CDMOs) is a Sponsor's major concern when...
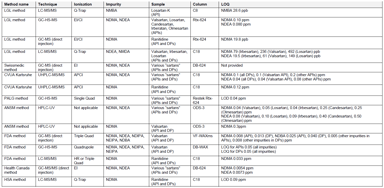
Analytical Methods for the Investigation of Carcinogenic Nitrosamines in APIs and Drug Products
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...
Quality & Compliance
Why is Process Optimization so Important in Cell and Gene Therapy Product Development?
A recent survey of experts from 145 cell and gene therapy (CAGT) companies revealed the ability to appropriately optimize the manufacturing process as their top concern. Red flags have been raised...

Top Challenges in Managing Your Product Quality Complaint Lifecycle
The Product Quality Complaint program is an essential tool in a company’s quality and compliance toolkit, not only for reducing patient risk and enhancing customer satisfaction, but because it...
Quality & Compliance
The Importance of Using a Multidisciplinary Approach in Early-Stage Development for Cell and Gene Therapy Products
Implementing a multidisciplinary approach in cell and gene therapy product development is critical to the product’s eventual success or failure. In our experience, the consequence of not effectively...
Phase Appropriate Controls and GMPs in Cell and Gene Therapy
How much is too little versus too much when developing quality systems and controls for investigational cell and gene therapies? Because these therapies are being administered to patients during all...
7 Tips to Help You Prepare for Compliance in a Post COVID-19 World
Yes, there is a light at the end of the long, dark COVID-19 tunnel, and people’s lives will return to a state of normalcy. However, what will the new state of normalcy look like in a post COVID-19...

Quality & Compliance
12 Critical Questions and Answers for a Successful Tech Transfer
12 Critical Questions and Answers for a Successful Tech Transfer Now, more than ever, companies are transferring products and processes from one site to another, often facing pressures on time,...
Quality & Compliance
Three Things 2021 Has in Store for Pharmaceuticals
Now that we have wrapped up the year 2020, it’s important to look ahead and prepare for upcoming regulatory deadlines. It is essential to address these changes and develop a plan, albeit nimble as...
Quality & Compliance
Meet the Expert: Eric Good, PhD
ProPharma has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Analytical Method Transfer Checklist
As you plan for an upcoming Tech Transfer, have you considered if you are appropriately prepared to conduct an analytical method transfer? With the simple analytical method transfer checklist...
Meet the Expert: Robert Beall, PMP
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Quality & Compliance
Is Your Technical Transfer Process Aligned with Process Validation Requirements?
There has been a lot of discussion recently concerning process validation and technology transfer, including utilizing virtual technology transfers to quickly move products through the development...

Meet the Expert: Daniel Wong
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Regulatory Sciences
4 Common Bottlenecks to Avoid in the Development of Biopharmaceuticals
If you are involved in early development of biopharmaceuticals, have you ever experienced serious delays because of problems arising from tech transfer, from the first pilot scale batches not meeting...