Quality & Compliance
Top Questions & Answers for Managing the New IVDR
With an increased need for high quality in vitro diagnostic medical devices (IVDs), the In Vitro Diagnostic Regulation (IVDR, 2017/746) was entered into force for all IVDs in 2017 with a five-year...
Should You Undergo Virtual Factory Acceptance Testing?
Although domestic travel is slowly ramping up, companies may still consider virtual factory acceptance testing (FAT). FAT is an optional step in the life sciences’ process of purchasing factory...
A Roadmap for Clinical Trials: QP Certification of IMP
Please note that updates to regulations may have been implemented since the publication of this article. The 20th century was the century of many scientific discoveries in the field of medicine....
eQMS: Your Questions, Answered
If you work in a regulated industry, you’ve most likely heard the term eQMS or enterprise quality management system. But you may be wondering what is it? Why do I need it? And how do I choose the...
How to Adopt an eQMS in 3 Simple Steps
A recent survey showed that 33% of the organizations surveyed use paper quality management systems; 60% use some paper and some digital; and 7% use no QMS yet. (source: Gartner peer insights) Quality...
Implementing eQMS in a Regulated Environment
Everything we do today is traceable. The integrity of the data, documents and decisions needs to be defendable and consistent. Electronic Quality Management Systems (eQMS) are an effective way to...
Meet the Expert: Maurice Weijers
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Quality & Compliance
Development with the End in Mind – Overcoming Early-Phase Challenges
A drug must be safe and effective. Manufacturing of a drug product should consistently yield a predetermined quality. These are the undisputed goals for commercialization of a drug within our...
Quality & Compliance
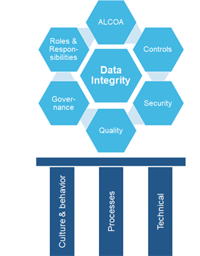
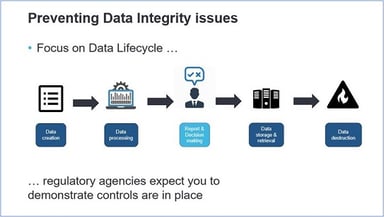
5 Step Plan for Data Integrity Compliance
Are you always ready to be inspected for Data Integrity (DI) activities in your facility? Are compliance and data integrity aspects implemented in your organization’s QMS? Are the systems in your GxP...
Quality & Compliance
Five Steps Toward a Mature Data Integrity Culture
The corporate and quality culture has a significant effect on the maturity level of Data Integrity within a regulated company and should, therefore, be assessed and understood. To achieve the level...

Meet the Expert: Bram Lardée
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
How to Implement an Effective Audit Trail
Maintaining an audit trail is a regulatory compliance requirement, but what makes an audit trail beneficial for maintaining effectiveness and complying with regulations? This blog will explain what...
Quality & Compliance
Prepare for Your Next Audit: A 5-Point GMP Checklist
Ensuring you have full control over your processes, facility, and quality management system (QMS), and ultimately your final product quality, is a demanding and important task. An inability to do so...
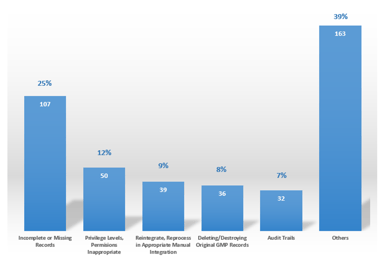
Measuring the Maturity of Data Integrity
We live in a world of data: there’s more of it than ever before, in a ceaselessly expanding array of forms and locations. Besides this, most people in their organizations are not always aware of data...
Regulatory Sciences
Life Science Outsourcing: Not sure you’re ready to turn over the keys?
It's no secret. To be successful in the life sciences industry you must accomplish a lot – achieve and maintain profitable growth, deliver safe and effective products to patients quickly, manage...