Quality & Compliance
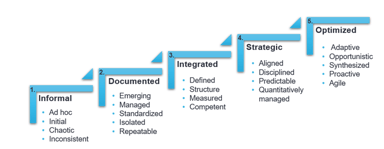
Improve Quality Using an Organizational Maturity Model
If this is your first introduction to an Organizational Maturity Model (OMM), you may have a few questions. What is an OMM? What are some common obstacles I might face when implementing an OMM? How...
Regulatory Sciences
Understanding EMA and FDA Regulations on Nitrosamine Control
On September 26, 2019, the European Medicines Agency (EMA) released an advice to Marketing Authorization Holders (MAH) of human medicines to review their drug products on possible presence of...
Quality & Compliance
Are Your Compliance Obligations Being Properly Upheld? Avoid This Common Outsourcing Mistake!
Over the past several decades, the traditional approach to drug development and manufacturing has expanded to include the outsourcing of a range of functions from product development and testing, to...
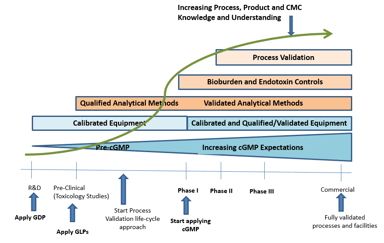
Pharmaceutical Tech Transfer Best Practices - A Quality Perspective
About 15 years ago, I was a project management director responsible for moving monoclonal antibodies (MABs) from Phase III clinical to commercial manufacturing. I had the distinct pleasure of working...
Unlocking Success with Clinical Trial Safety Monitoring During a Pandemic
Earlier this year I wrote to you about US FDA March 2020 issuance of a new guidance for industry, Investigators, and Institutional Review Boards regarding the conduct of clinical trials during the...

Quality & Compliance
Uncover Opportunities for Improvement with an Annual Product Review
The Annual Product Review (APR), also known as the Annual Product Quality Review (APQR), is required for marketed products in an FDA-regulated environment. You may ask, "Why would I want to perform...
Quality & Compliance
EudraLex Volume 4, Annex 1 Update: What You Need to Know
EudraLex Volume 4, Annex 1 provides guidance for the manufacturing of sterile medicinal products that are intended for the European market. It has been updated several times, with the latest revision...
How to Prepare for Laboratory Partner Selection during CBD Product Development
The interest in developing consumer products or therapies derived from Cannabis or CBD is continuously growing. As these new products come to market, there is increasing need to comply with...
Quality & Compliance
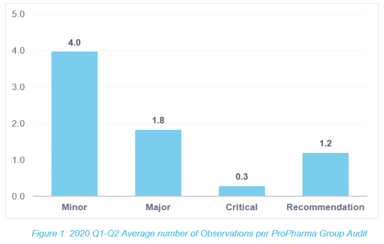
How Many Observations are Hiding in Your Quality System?
Reflecting on 2020, we have become accustomed to the global shift into a world of virtual, remote, restricted, and paused. One no longer flinches when hearing that something has been modified,...
6 Tips to Prepare Your Medical Cannabis Facility for Inspection
You may be considering building a new facility for growing, harvesting, and processing medical cannabis, or perhaps you have an existing facility and want to export to the European Union. What should...
3 Basic Data Integrity Principles to Protect Value and Drive Success for Cell and Gene Therapy/ATMP Development
Cell and Gene Therapy (CGT)/Advanced Therapy Medicinal Products (ATMPs) have the incredible potential to cure devastating illnesses, such as cancer, on a more personalized level. But, due to the...
Quality & Compliance
Meet the Expert: Simona Mills, PMP
ProPharma has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
6 Ways to Increase the Value of Your Cell and Gene Therapy or ATMP Development
As the field of modern medicine is changing, so should the development strategies of these new therapies such as cell and gene therapy (CAGT) products, also known as advanced therapy medicinal...

Quality & Compliance
EMA vs. FDA Virtual GCP Auditing Guidance: What You Need to Know
In response to the COVID-19 pandemic, global regulatory authorities adopted a pragmatic virtual auditing approach. This approach includes the flexibility and procedural simplifications to maintain...
Quality & Compliance
Virtual GCP Auditing: Your Questions Answered!
How quickly the auditing landscape has changed! Less than one year ago if ProPharma were asked to perform a clinical audit on your firm’s behalf, we would reply with "when, what, and where?" Today...
Quality & Compliance
Key Takeaways: New Draft Guidance on Cannabis and Clinical Research
On July 21st, 2020, the FDA released a draft guidance document for developers of cannabis and cannabis derived compounds, aptly titled “Cannabis and Cannabis Derived Compounds: Quality Considerations...