Meet the Expert: Ewelina Czerniec-Michalik
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to support...
Quality & Compliance
Establishing Process Equivalency
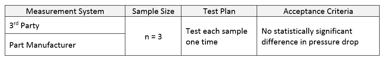
A common question when transferring an established process from one facility to another is how to establish that the transferred process is performing equivalently to the original process. Sounds...
Quality & Compliance
How to Safely Launch Medical Cannabis Products in Germany
Over the past few weeks the withdrawal of cannabis products from the German market has been a topic which generated a lot of publicity. Therefore, we want to share some tips on how to safely launch...
Three Steps Towards Complying with Nitrosamine Regulations
All Marketing Authorization Holders (MAH) of medicines for human use are facing what might feel for many, as a new requirement: review their drug products on the possible presence of nitrosamines....
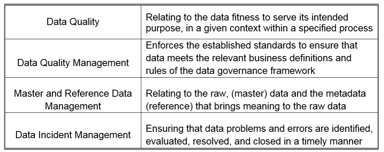
Just How Mature Is Your Data Lifecycle / Data Management Function?
The attention of regulatory agencies continues to focus on data integrity, as observed by the increase of FDA observations over the course of the last few years. Having a proper data lifecycle / data...
Quality & Compliance
Get the Most Out of Your GMP Effectiveness Checks
We work in a highly regulated industry. Whether you are associated with the manufacturing of a drug, a biologic, or a device, you understand the importance of those regulations on the safety and...

Deviations: Beyond the Basics
There are plenty of guidelines and instructions on implementing a deviation system in a pharmaceutical/medical device company. However, there is a big difference between theory and practice when it...
Quality & Compliance
FDA's Top 483 Observations for 2018: A Reflection of Industry’s Compliance
At the beginning of each federal fiscal year, the US FDA posts the previous year's Form 483 observation metrics issued by each product center. Inspections ending between 10/1/2017 and 9/30/2018, for...
Utilizing an Agile Framework When Implementing ATMPs
Who says you can’t teach an old dog a new trick? Having spent the last 25+ years in small molecule, large molecule, medical devices, I have spent a lot of time planning and executing everything from...

Health Apps and the Requirements Imposed By the Law
If you check Apple’s App Store or Google’s Play Store you will find an overwhelming list of health and fitness apps. This list only gets longer, if you include the number of people who use these...
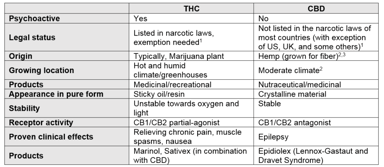
7 Things to Consider in Medicinal Cannabis Development
Mention the word cannabis and the confusion starts; legal or illegal, nutraceutical or medicinal product, psychoactive or non-psychoactive, clinically significant or not. At the same time, the...
Innocent Until Proven Guilty: Hypothesis Test
Each of us makes important decisions that shape our lives, every day. We try to make the best decisions possible and yet, as the adage goes "all predictions of the future are wrong, some are just...
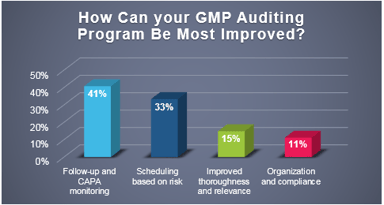
Industry Poll: How Can Your GMP Auditing Program Be Most Improved
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...
The Important Role of Advanced Therapy Medicinal Products
Increasing safe and effective patient treatment opportunities for the future is one of the driving forces behind ProPharma Group’s business. It’s also the driving force behind why many individuals...
Quality & Compliance
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...