If this is your first introduction to an Organizational Maturity Model (OMM), you may have a few questions. What is an OMM? What are some common obstacles I might face when implementing an OMM? How can an OMM benefit my organization? Here are your questions answered…
OMM is a common MBA coursework topic and that is where I was first introduced to the concept and process. Although OMM is used in many functional areas such as Project Management, Leadership, and Personnel Management, in this article, we will address improvements specific to Quality.
What is an Organizational Maturity Model?
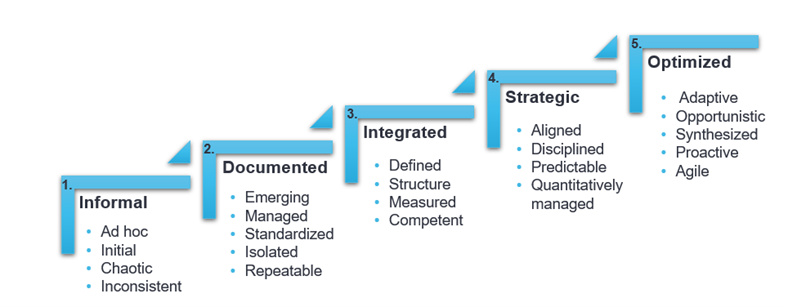
An Organizational Maturity Model is a collection of reliable, proven processes focused on a specific discipline. The five-step framework ranges from basic to sophisticated practices. Organizations, people, and departments are objectively rated on a scale of 1-5 and given a score. The organization or department is evaluated on the scale, represented as such:
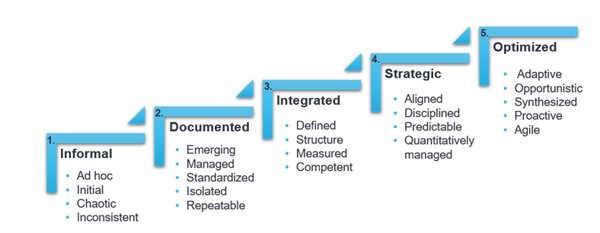
If we take your current Quality Unit for example, are they chaotic and inconsistent? If yes, they would be rated a one (1). If they had structure, were well-defined, and competent, they would be rated a three (3). The improvement process is how you advance from a one to a three. Now you have a basic understanding of an OMM!
What Are Common Obstacles?
Given the example above, what are some common obstacles with implementing an OMM? One of several obstacles is that the Quality Unit may not have an existing process-improvement model, or even a basic understanding of what processes need improvement.
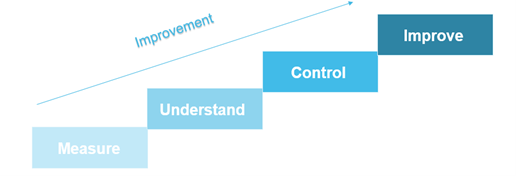
As James Harrington said in The Improvement Process, “If you can’t measure it, you can’t understand it. If you can’t understand it, you can’t control it. If you can’t control it, you can’t improve it.”
Other obstacles may include:
- Best practices are not widely used
- Previous investment in initiatives with little return on investment
- A struggle to maintain capabilities amidst turnover, backlogged work, and retirements
Another large barrier that is found within organizations, and certainly Quality departments, is many competency assessments focus on the person… while organizational maturity assessments focus on practices and processes. Think about it this way: When something goes poorly and a corrective action is required, do we often blame the person first and readily discount the practices or processes that the person follows. If so, therein lies a barrier to implementing an OMM.
What Are the Benefits?
Now that we have reviewed a few of the implementation challenges, let us look beyond that at the benefits of OMM and more specifically, as it applies to improving Quality. In the chart below, take a moment to review all five levels of an OMM, with one being the lowest and five being the highest. Where would you put either the quality of your product or your Quality Unit?

Pretty grim or pretty good? Do not feel alone if the rating was low. The majority of organizations or departments fit into either level 1 or 2, while about 25 percent of organizations find themselves at level 3. Very few organizations start off at level 4 of 5. Let us move forward with the assumption that your Quality Unit is a level 1 and wants to be a level 3. What are the benefits of such a change?
Implementing an OMM allows the Quality Unit to take an inventory of their current capabilities and set a baseline for measuring improvement. Implementation documents the need for change and establishes a common language and shared vision. Overall, implementing an Organizational Maturity Model sets the stage for greater organizational improvement.
Improving Quality at your organization is a worthy goal, but you don’t have to do it alone. Contact our team today to learn how ProPharma can help you get started.