
Clinical Research Solutions
Compounded drug search added to FDA’s NDC directory webpage
FDA recently added a search function to the National Drug Code (NDC) Directory webpage for human drugs compounded by outsourcing facilities that assign NDC numbers to their products. This update was...
Meet the Expert: Markus Ganzlin
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

EMA appoints Chief Medical Officer
June 1, 2022 Steffen Thirstrup has been appointed as Chief Medical Officer of EMA. In this role, he will provide scientific leadership across EMA and its scientific committees to reinforce the...

Clinical Research Solutions
FDA Proposes New Rule for Distribution Compliance
FDA has announced the availability of a proposed rule National Standards for the Licensure of Wholesale Drug Distributors (WDDs) and Third-Party Logistics Providers (3PLs). The proposed rule sets...
Clinical Research Solutions
How to Avoid Common Pitfalls in the Development of Biosimilars
As many blockbuster biologicals face the expiration of their patents, so-called "patent cliff", many biotech businesses direct their attention to the field of biosimilars. The development of...

Clinical Research Solutions
Why Partner with a CRO that has In-House DCT Capabilities?
When you need to outsource your clinical trials and decentralized visits, it can be beneficial to select a single provider that can do both. There are many synergies that come from using a single...
Clinical Research Solutions
7 Questions to Ask When Selecting a DCT Provider
When considering a decentralized approach to your clinical trial, it can be confusing or overwhelming to identify a provider that will truly understand and support specific study needs. ProPharma...

Clinical Research Solutions
4 Steps to Decentralize Clinical Trials
Decentralized Clinical Trials (DCTs) is a buzzword in the clinical research space that has been gaining notoriety because of the COVID-19 pandemic. It’s a concept that revolves around bringing...

Clinical Research Solutions
FDA's Expedited Programs Explained
In order to incentivize the development of therapies (drugs biologics) to fill unmet medical needs for treatment of serious conditions, the FDA has developed various programs to expedite drug...
Roadmap for Successful IVDR Transition, Part III: Project Management
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching in May 2022. In this blog series, we discuss the final months before the IVDR date of application, how to...
Why You Need a Decentralized Clinical Trials Strategy - Before Disaster Strikes
Imagine having your clinical trial ready to go, or already underway, and overnight the brakes are slammed, bringing your trial to a screeching halt. This was the reality for sponsors around the world...
What the IVDR Is and How to Prepare
In May 2022, the IVDD will be repealed by the European Committee, thereby ending the transition period. To ensure you're compliant with IVDR by that date, learn everything you need to know about the...
Clinical Research Solutions
Roadmap for Successful IVDR Transition, Part II: Technical Documentation & Software
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching (May 2022). In this blog series, we discuss the final months before the IVDR date of application along with...
Clinical Research Solutions
FDA’s Breakthrough Therapy Designation vs PRIority MEdicines (PRIME) Application in Europe
What are Breakthrough Therapy Designation and PRIority MEdicines (PRIME) Applications? The advancement of modern medicine, and the accessibility of researched and regulated medication, has greatly...
Clinical Research Solutions
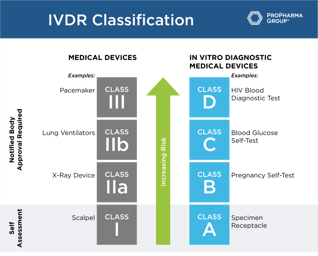
Roadmap for Successful IVDR Transition
Roadmap for Successful IVDR Transition: The compliance dates for the In Vitro Diagnostics Regulation (IVDR) will become effective on May 26, 2022. To help you with the IVDD to IVDR transition, we've...
Clinical Research Solutions
Top Questions & Answers for Managing the New IVDR
With an increased need for high quality in vitro diagnostic medical devices (IVDs), the In Vitro Diagnostic Regulation (IVDR, 2017/746) was entered into force for all IVDs in 2017 with a five-year...