
Clinical Research Solutions
MoCRA 2022: Updated Requirements for Cosmetic Companies
MoCRA enacts the most significant expansion of the US FDA to regulate cosmetics since 1938. After 85 years of effectiveness the Modernization of Cosmetics Regulation Act of 2022 or "MoCRA" was...

Rare/Orphan Diseases and the African American Community
How do we improve outcomes for an uncommon healthcare challenge in a community challenged with common healthcare issues? The Orphan Drug Act defines a rare disease as a disease or condition that...

The Boston ATMP Biotech Conquest
After panning over the Boston Business Journal and seeing yet another local VC (Venture Capital) firm raise a record $350 million in venture capital for Massachusetts life science, I asked myself:...

Clinical Research Solutions
ProPharma Group's Dr David Crome to Act as Compliance Monitor to the MHRA
From April 2022, the MHRA has been developing a pilot programme for GMP and GDP remediation supervision by eligible consultants acting as Compliance Monitors (CM) on behalf of companies that have...

Clinical Research Solutions
Your Vendor Audit Program: On-site or Remote / Virtual?
It is quite common that a sponsor company will outsource services to external vendors, whether for additional expertise, remote locations, or simply due to lack of availability of resources within...
Clinical Research Solutions
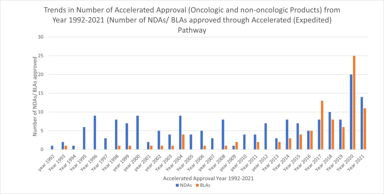
FDA Accelerated Approval Pathway: A Potential Missed Opportunity for Sponsors
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...

Clinical Research Solutions
Clinical (IMP) Drug Supply…It's Complicated
Things to consider and how to ease the process IMP Supply Management is a journey where GMP, GCP, and GDP meet. This journey includes finance, flow of products, and documentation. How a company...
Clinical Research Solutions
What You Need to Know About Developing Vaccines
An unlikely beacon of hope from the otherwise disastrous Covid pandemic, may come in the form of renewed attention towards approaches to vaccine development. The Importance of Vaccines The...
%20VII%20and%20Type%20D%20Meetings-%20A%20New%20Mechanism%20for%20Interacting%20with%20FDA.jpeg?width=384&height=256&name=Prescription%20Drug%20User%20Fee%20Act%20(PDUFA)%20VII%20and%20Type%20D%20Meetings-%20A%20New%20Mechanism%20for%20Interacting%20with%20FDA.jpeg)
Prescription Drug User Fee Act (PDUFA) VII and Type D Meetings: A New Mechanism for Interacting with FDA
For those who have been awaiting Congressional reauthorization of PDUFA, the wait is over. On September 30, 2022, the President signed into law the FDA User Fee Reauthorization Act of 2022. We...

What You Need to Know About CBER Pre-IND Meetings
The FDA provides several opportunities to hold meetings with Sponsors to gain clarification and agreement on the development of medicinal products. At the preliminary stages of development, one such...
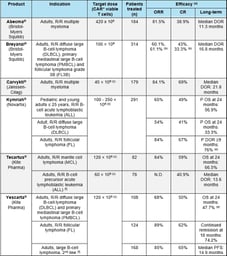
CAR-T Cells: Challenges, Lessons Learned, and Guidance for the Clinical Development
It comes as no surprise to any pharmaceutical or biotech company that planning the clinical development of CAR-T cells is an extremely challenging endeavor: high efficacy is expected in each targeted...

Clinical Research Solutions
How to Comply with the Nitrosamine Regulations for Your New Drug Product Marketing Applications
Are you in the development phase for your medicinal product? Have you assessed your manufacturing processes with respect to the requirements for investigating the potential presence of nitrosamine...

Clinical Research Solutions
USP and FDA Propose Updates to Good Storage and Distribution Practices
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...
Clinical Research Solutions
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Clinical Research Solutions
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...
Clinical Research Solutions
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...