Should You Undergo Virtual Factory Acceptance Testing?
Although domestic travel is slowly ramping up, companies may still consider virtual factory acceptance testing (FAT). FAT is an optional step in the life sciences’ process of purchasing factory...
Regulatory Sciences
Regulatory Strategy for Clinical Trials in the European Union
Setting up a clinical trial in the European Union (EU) has historically been an expensive and time-consuming business. With 27 individual member states each requiring its own review and approval, it...
Regulatory Sciences
Is it Time to Sharpen Your Target Product Profile (TPP)?
Is it Time to Sharpen Your Target Product Profile (TPP)? We’ve heard it countless times: "Fail early and live to fight another day." "Preserve capital, human resources, and energy for a project with...
Clinical Research Solutions
7 Benefits of Working with a Biostatistician
Biostatisticians have become increasingly important as many data-driven public health projects and clinical trials have expanded. So, what exactly does a biostatistician do? These individuals are...
A Roadmap for Clinical Trials: QP Certification of IMP
Please note that updates to regulations may have been implemented since the publication of this article. The 20th century was the century of many scientific discoveries in the field of medicine....
Clinical Research Solutions
Understanding Bioequivalence and Product-Specific Guidances
The FDA regularly issues new and revised product-specific guidances to facilitate the availability of generic drugs and assist the generic pharmaceutical industry with identifying the most...

Regulatory Sciences
European Expedited Regulatory Programs Explained
Do you really know how to accelerate the approval of your innovative product in Europe? The FDA's incentives for promising new medicines are widely known. Accelerated approval, priority review, fast...
Meet the Expert: Maurice Weijers
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Clinical Research Solutions
Four Benefits of Requesting an FDA Pre-IND Meeting
Although not required, a Pre-IND Meeting is a critical milestone, one that is highly recommended by the FDA. The goal of the meeting is to receive general agreement from FDA that your drug...
Clinical Research Solutions
EMA Takes Steps to Minimize Animal Testing During Product Development
On Wednesday, September 29th, EMA announced the implementation of new measures indented to replace, reduce, and refine animal use in the development, manufacturing, and testing of human and...
Clinical Research Solutions
Critical Issues in Drug Development: Don't Get Derailed in Developmental Efforts
Pharmaceutical companies spend massive amounts of money on drug development. Regardless of a firm’s size, the investment is substantial; the smallest error can have catastrophic consequences,...
Clinical Research Solutions
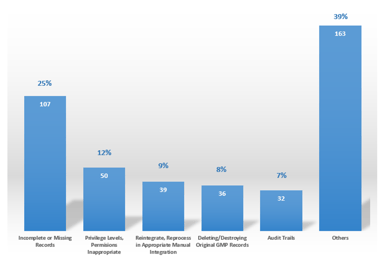
Five Steps Toward a Mature Data Integrity Culture
The corporate and quality culture has a significant effect on the maturity level of Data Integrity within a regulated company and should, therefore, be assessed and understood. To achieve the level...
Clinical Research Solutions
How to Present Scientific Data That Generates Regulatory Approvals
Today’s patients benefit from having access to the safest, most advanced pharmaceutical systems around the world. This is because of the work done by the various regulatory bodies who govern the...
6 Benefits of Custom PMO Design
A Project Management Office ("PMO") can be incredibly advantageous to large companies with many ongoing projects across various job sites. A PMO streamlines project management processes to make a...
Clinical Research Solutions
Life Science Outsourcing: Not sure you’re ready to turn over the keys?
It's no secret. To be successful in the life sciences industry you must accomplish a lot – achieve and maintain profitable growth, deliver safe and effective products to patients quickly, manage...
Clinical Research Solutions
Understanding The Medical Device Single Audit Program
The Medical Device Single Audit Program (MDSAP), initiated by the International Medical Device Regulators Forum (IMDRF), created a global approach to auditing and monitoring the manufacturing of...