Clinical Research Solutions
GAMP® 5 Basis for Quality System Compliance
Next week I’ll be in San Francisco conducting an ISPE training class on Risk Based Approach to GxP Process Control Systems. Attendees will include employees from a variety of regulated life cycle...
Clinical Research Solutions
Process Validation: Gate Eight of the Nine Gate Transfer Process
Today we have the next phase of the Nine Gate Transfer Process: Gate 8 Process Validation. To review the previous steps before continuing, examine Gates 1-7 here: Gate 1: Assessment Gate 2:...
Clinical Research Solutions
Evaluating the Effectiveness of a Corporate Compliance Program: How Does Your Program Measure Up?
At the recent Pharmaceutical Compliance Congress (PCC), Zane Memeger, U.S. Attorney for the Eastern District of Pennsylvania stated that one of the key questions that a company should ask about its...
Rethinking the OTC Monograph System
When the OTC drug review was undertaken in the 1970s, it was deemed the best solution available at the time for efficiently assessing the safety and efficacy of the countless drug products being sold...
Clinical Research Solutions
To Requalify or to Continuously Monitor, That is the Question
Your manufacturing equipment and processes have been established, qualified and validated; all you have to do now is rake in the profits right? Think again, the process of maintaining the validated...
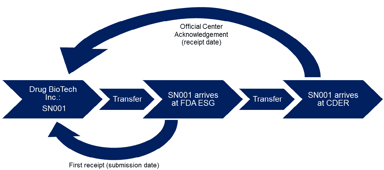
Words, Words, Words: The Importance of Diction in Regulatory Submissions
In continuation of its series of guidances on electronic submissions, FDA recently released a guidance for receipt dates of electronic submissions which emphasizes one important fact: word choice...
Clinical Research Solutions
What’s the Skinny on Dietary Supplements?
One doesn't have to spend much time in a pharmacy, grocery store or even a typical American house to see that dietary supplements have found a place in people's lives. In fact, roughly 54% of U.S....
Clinical Research Solutions
Understanding Statistical Intervals: Part 3 - Tolerance Intervals
We saw in Part 1 of this series how a confidence interval can be calculated to define a range within which the true value of a statistical parameter such as a mean or standard deviation is likely to...
Clinical Research Solutions
The $142 Million Question: Will the Kaiser Judgment Encourage More Private Payor Actions for Off-Label Promotion?
If enforcement against pharmaceutical companies for off-label promotion by the Office of Inspector General (OIG) and the Department of Justice (DOJ) and the Food & Drug Administration (FDA) were not...
Clinical Research Solutions
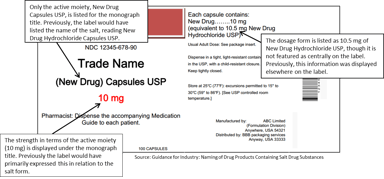
FDA Guidance Follows USP Salt Policy & Aims to Make Conversions Between Salt Forms Simpler
The Food and Drug Administration (FDA) recently published a draft guidance that outlines the “Naming of Drug Products Containing Salt Drug Substances” in accordance with the recently implemented USP...
Clinical Research Solutions
Not a Bitter Pill to Swallow: FDA Releases Guidance on Size and Shape of Generic Drugs
In a Federal Register notice to be published on Dec. 10, 2013, FDA announced the release of a new guidance titled “Size, Shape and Other Physical Attributes of Generic Tablets and Capsules.” The...
Clinical Research Solutions
OIG Signals Importance of Board-Level Compliance Committee in J&J Corporate Integrity Agreement
In the just issued Johnson & Johnson Corporate Integrity Agreement (CIA), the Office of Inspector General (OIG) has, for the second time in less than a year, required that a company maintain a...
Clinical Research Solutions
Government Aims its Sights on Smaller Pharmaceutical Companies
Big pharma and their deep pockets have been the focus of enforcement activities for a number of years but in 2013 that tide may be turning. In the past few months, settlements with ISTA...
Clinical Research Solutions
Three Solutions to Typical Autoclave Temperature Mapping Conundrums
Temperature Mapping is an intrinsic part of equipment validation. It evaluates the quality and compliant nature of the equipment to ensure the equipment meets the user and regulatory agency’s...
Clinical Research Solutions
Personalized Medicine – How Will the Batch Size Change Our Lives?
Hardly a week goes by without the announcement of a new scientific break-through in science and medicine that promises safer, more efficacious and personalized medicine. Where are all these...
Clinical Research Solutions
Lean CSV – How to Reduce Waste and Increase Value
What is lean? Lean is a business system focused on continuously improving processes by reducing the time taken and the waste involved in delivering increasing value to the customer. Customers in this...