Regulatory Sciences
Monoclonal Antibody Sameness - Effects on Orphan Drug Development
We note with interest FDA’s recently released Guidance for Industry: Interpreting Sameness of Monoclonal Antibody Products Under the Orphan Drug Regulations (April 2014). The guidance summarizes the...
Regulatory Sciences
FDA Thinking on Biosimilar Immunogenicity Issues - A Case Study Based on the Enoxaparin ANDA Approval
Following extensive marketing of Lovenox® (enoxaparin sodium), a low-molecular weight heparin, a number of biosimilar versions of enoxaparin and one ANDA-approved formulation (enoxaparin sodium,...
Regulatory Sciences
What Can Testosterone Gels Teach Us About Abuse-Deterrent Opioids?
For most drugs, the process of developing and obtaining FDA approval of a generic version is simple and well-defined: sponsors must only prove that their product is pharmaceutically equivalent and...
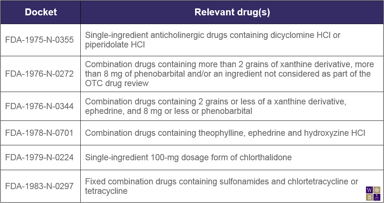
Rethinking the OTC Monograph System
When the OTC drug review was undertaken in the 1970s, it was deemed the best solution available at the time for efficiently assessing the safety and efficacy of the countless drug products being sold...
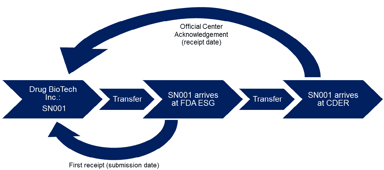
Words, Words, Words: The Importance of Diction in Regulatory Submissions
In continuation of its series of guidances on electronic submissions, FDA recently released a guidance for receipt dates of electronic submissions which emphasizes one important fact: word choice...
Regulatory Sciences
FDA Issues a Reminder on Excipients in Dietary Supplements
In the new guidance on substances that can be added to foods, including beverages and dietary supplements, the FDA reminds manufacturers that the excipients added to oral dosage forms of these...
Unapproved Codeine Products and Some DESI Drugs need FDA Approval or Cease Marketing
In a Federal Register notice published today, the Food and Drug Administration (FDA) announced its intention to take enforcement action against misbranded or unapproved prescription products...
Regulatory Sciences
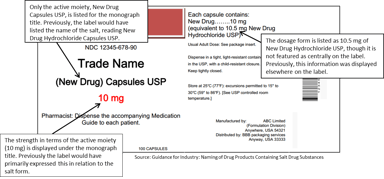
FDA Guidance Follows USP Salt Policy & Aims to Make Conversions Between Salt Forms Simpler
The Food and Drug Administration (FDA) recently published a draft guidance that outlines the “Naming of Drug Products Containing Salt Drug Substances” in accordance with the recently implemented USP...
Regulatory Sciences
Not a Bitter Pill to Swallow: FDA Releases Guidance on Size and Shape of Generic Drugs
In a Federal Register notice to be published on Dec. 10, 2013, FDA announced the release of a new guidance titled “Size, Shape and Other Physical Attributes of Generic Tablets and Capsules.” The...
Regulatory Sciences
FDA Proposes Controversial Rule Allowing ANDA Applicants to Change Drug Labels
On Nov. 13, 2013, the Food and Drug Administration (FDA) published a Proposed Rule in the Federal Register. The title of the Proposed Rule is “Supplemental Applications Proposing Labeling Changes for...
Waivers for Carcinogenicity Studies? Not So Fast!
The recently published request for comments regarding the Proposed Change to Rodent Carcinogenicity Testing of Pharmaceuticals signals intent by regulatory agencies to alter what Sponsors consider in...

FDA Releases New Guidance on Meetings with Sponsors of Biosimilars
On March 29, 2013, the FDA made available a draft guidance for Sponsors of biosimilar products outlining the procedures and processes for meetings with the FDA. Although much of guidance for the...

Regulatory Sciences
Generic Drug User Fee Act (GDUFA) Overview
FDA user fees have been a fact of life for regulated industries for nearly two decades. As you continue to comply by paying the fees, you should understand what the revenue does for you. In this...
Regulatory Sciences
FDA Comments on Proposed Prescription Drug Labeling
Standardized prescription drug labeling was implemented by FDA in 1979. In the following years as labeling became more complex, FDA re-evaluated its usefulness and published a final rule in 2006...
Regulatory Sciences
Extended Review Timeline for Certain Applications under Proposed PDUFA V
The Prescription Drug User Fee Act (PDUFA) is due to be reauthorized by the end of September this year. Along with the usual increases in fees, the latest version of the act (PDUFA V) includes some...
Regulatory Sciences
Label Changes Related to the QTc Interval
In a recent FDA Drug Safety Communication, the Agency announced that the Celexa (citalopram hydrobromide) label now has revised dosing recommendations based on evaluations of post-marketing reports...