Clinical Research Solutions
Brexit: New Regulatory Opportunities in the United Kingdom
As the dust settles on the final Brexit deal, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) is forging new opportunities to innovate, increasing its efficiency, and...
Clinical Research Solutions
How GDUFA II Impacts the Timing and Approval Process for Generic Drug Sponsors
How GDUFA II Impacts the Timing and Approval Process for Generic Drug Sponsors: Facing several regulatory challenges related to the review of abbreviated new drug applications (ANDAs), Congress first...

Clinical Research Solutions
12 Critical Questions and Answers for a Successful Tech Transfer
12 Critical Questions and Answers for a Successful Tech Transfer Now, more than ever, companies are transferring products and processes from one site to another, often facing pressures on time,...
Clinical Research Solutions
Meet the Expert: Eric Good, PhD
ProPharma has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Top 9 Failure Points During Nonclinical Development
The process of drug development involves clinical and nonclinical studies. Nonclinical studies are considered crucial for understanding the safety of new drugs. Before testing a drug in people,...
Quality & Compliance
Analytical Method Transfer Checklist
As you plan for an upcoming Tech Transfer, have you considered if you are appropriately prepared to conduct an analytical method transfer? With the simple analytical method transfer checklist...

Clinical Research Solutions
Stability Testing of New Drug Substances and Products
The stability of a drug substance or product is a critical attribute for all pharmaceutical products. As such, stability testing is required throughout the drug development phase as well as...
Data Integrity in Clinical Research: Audit Trail Review as a Key Tool
Data Integrity in Clinical Research: Audit Trail Review as a Key Tool A recent position paper from the eClinical Forum and the Society for Clinical Data Management highlighted the value of audit...
Clinical Research Solutions
The Truth Can Hurt - But Hearing It at the Right Time Can Save Time and Money
No one has an ugly baby. At least, no one thinks their baby is ugly. Every new parent thinks their baby is the most beautiful baby of all time. But the unfortunate fact is that there are ugly babies....
Clinical Research Solutions
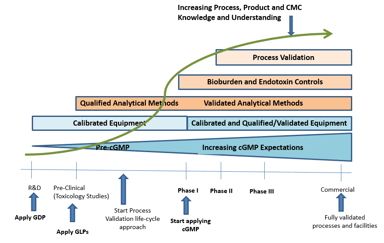
Is Your Technical Transfer Process Aligned with Process Validation Requirements?
There has been a lot of discussion recently concerning process validation and technology transfer, including utilizing virtual technology transfers to quickly move products through the development...
Clinical Research Solutions
5 Tips for Achieving Regulatory Success in 2021
As we begin to wrap up the year and look ahead to 2021, it is critical to prepare your team for what is coming in the weeks and months that lay ahead. Preparation and preparedness are key to ensuring...
Clinical Research Solutions
A Guide to the Clinical Study Report
What is a Clinical Study Report? A Clinical Study Report (CSR) is a document that describes the methods and results of a clinical study or trial, along with a short discussion of key findings related...
Clinical Research Solutions
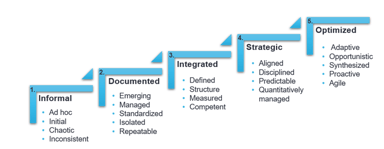
Improve Quality Using an Organizational Maturity Model
If this is your first introduction to an Organizational Maturity Model (OMM), you may have a few questions. What is an OMM? What are some common obstacles I might face when implementing an OMM? How...
Clinical Research Solutions
Understanding EMA and FDA Regulations on Nitrosamine Control
On September 26, 2019, the European Medicines Agency (EMA) released an advice to Marketing Authorization Holders (MAH) of human medicines to review their drug products on possible presence of...
Clinical Research Solutions
The Role of Clinical Data and Clinical Data Science
Clinical data and its analysis are critical to clinical research. Ensuring the overall quality of clinical data is then paramount to ensuring quality care and appropriate decision-making in the...
Clinical Research Solutions
Are Your Compliance Obligations Being Properly Upheld? Avoid This Common Outsourcing Mistake!
Over the past several decades, the traditional approach to drug development and manufacturing has expanded to include the outsourcing of a range of functions from product development and testing, to...