Quality & Compliance
Does your Quality System stand up to the challenge?
Implementing and maintaining a Quality System is a complex challenge. It is as much as an art as it is a science. A company’s Quality System establishes the framework to manage and maintain...
Quality & Compliance
Top Takeaways from FDA's Guidance on Data Integrity
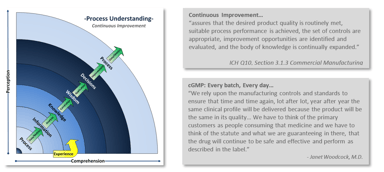
For those of you unaware, the FDA submitted a draft guidance on data integrity for comment in April. This is significant in that the last formal written guidance provided from FDA on this subject...
Quality & Compliance
CSV Considerations Around Data Integrity
Data integrity is a current hot topic, but not a new one, within the life sciences industries and associated product supply chains. This article will not delve into why there have been so many recent...
Quality & Compliance
Value Found in FDA Warning Letter Reviews
Starting with the issuance of a 483, the stepwise FDA enforcement process can be illustrated as follows: Given the seriousness of a Seizure and Injunction scenario, not to mention potential jail time...
Quality & Compliance
FDA Inspection? Rely on Your Vendors
If you’ve spent a reasonable amount of time in an FDA-regulated industry, you’ve most likely been part of an FDA inspection. Should you be more fortunate, you have participated directly with the...
Regulatory Sciences
The FDA’s Enforcement of Section 503B of the Federal Food, Drug, and Cosmetic Act
The FDA publishes weekly enforcement reports highlighting drugs that have been recalled during the previous week. Over the past several months these reports have been littered with hundreds of...
Quality & Compliance
Project Bullying
Believe it or not, project bullying in the workplace does exist. It is not a threat only at schools and on playgrounds. Project bullying can come for any source including the Project Manager, the...
Quality & Compliance
Are You Ready for AIQ? (Part II)
In the previous installment of this blog, we discussed the importance of establishing a Master Validation Plan, and how to plan and assess your validation approach. Today’s segment will complete the...
Quality & Compliance
Mergers, Acquisitions and Consolidations: Whose Validation Standards Do I Use?
If you are like the majority of those who have worked in the biotechnology and pharmaceutical industry for at least the last five years, your company has been part of a merger, acquisition or some...
Learn Your Lessons Well: How Regulatory Strategies and Clinical Trial Design Affect Approvability
A recent analysis by the Tufts Center for the Study of Drug Development places the price tag of bringing a new drug to market at around $2.6 billion. The reason for this is simple: failure. Drugs...
Quality & Compliance
PMP: The Benefit of Quality Knowledge
There are thousands of drug manufacturers that have a number of ongoing projects within Operations. Often times, these companies seek experienced consultants to assist/guide them to project...
Regulatory Sciences
Are you spending too much on lifecycle costs?
ASTM E2500 – Risk-based testing has been in play for several years now. By design, there is a significant opportunity to avoid many lifecycle costs without creating an adverse impact to quality or...
Why Are My Swab Recoveries So Low?
Cleaning Validation and GMP reviews of those protocols are challenging. They become even more challenging at a Contract Manufacturing Organization (CMO) where compliance assessments to in-house...
Quality & Compliance
Continued Cleaning Effectiveness: What Are You Doing? - Part II
My last blog focused on the industry trends concerning cleaning validation verification. Through a quick poll we found that out of twenty-three respondents, approximately fifty (50) percent are doing...
Quality & Compliance
Environmental Monitoring: The Top 3 Points to Consider
When a biopharmaceutical company builds new or renovates existing manufacturing space, the last consideration before turning the production key is the environmental monitoring of the same. This short...
FDA Updates Informed Consent Guidance
FDA recently updated its informed consent guidance in the form of an Information Sheet. The new document reflects the Agency’s current thinking on the informed consent process utilized in...