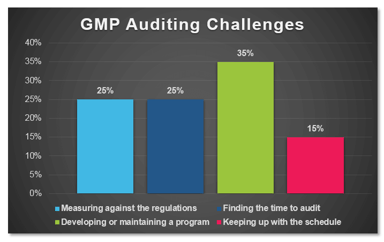
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
Quality & Compliance
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...

Quality & Compliance
Summary Considerations: Use of Electronic Records and Electronic Signatures in Clinical Investigations Under 21 CFR Part 11 – Questions and Answers
Here I provide some key summaries and considerations relative to FDA’s draft guidance that was submitted for review and comment in June 2017. If you don’t want to review the entire guidance, here are...
Quality & Compliance
Using a Matrix Approach to Media Fills in Sterile Compounding
A survey of FDA Form 483 observations issued to the 503B pharmacy industry reveals that outsourcing compounders are struggling to implement and manage compliant and risk-based approaches to aseptic...
Quality & Compliance
What Should Be on Your Clinical Trial Investigator Site Audit Checklist?
You live and operate in a regulated industry. Obviously, it’s crucial that you stay in compliance during your clinical trials. That’s because failure to do so has enormous and expensive consequences....

Quality & Compliance
Data Integrity 101: Is your data compliant?
Data integrity has recently been in the agency spotlight. In part because of the draft data integrity guidance issued April 2016, but primarily due to an increased number of inspection findings...
Quality & Compliance
Clinical Quality Systems & the Outsourced Model
The landscape of clinical trials is evolving. The changes that are happening are due to the increased number of FDA-regulated trials, as well as a rise in the complexity of clinical protocols. As...
Quality & Compliance
The Benefits of Process Characterization in Process Development
The desire for a robust and repeatable manufacturing process is shared by every organization that has a therapy or product in development and the only way to demonstrate that this desired state has...
Quality & Compliance
Understanding the 21st Century Cures Act: Part I
The recent passage of the 21st Century Cures Act (passed December 13, 2016) marks a significant milestone for medical device and drug development. I recently attended a meeting held by the Food and...
Quality & Compliance
#7: Quality Systems Approach to Pharmaceutical CGMP Regulations
In August 2002, the FDA announced the Pharmaceutical CGMPs for the 21st Century Initiative, which explained FDA’s intent of integrating quality systems and risk management approaches, and had a goal...
Regulatory Sciences
#10: Investigator Responsibilities – Protecting the Rights, Safety, & Welfare of Study Subjects
In October 2009, the FDA issued a guidance document regarding its expectations for investigators during the conduct of clinical investigations. The guidance, entitled Investigator...

Quality & Compliance
Is Your Laboratory PC Cloned From the Proper Image?
Managing compliance for computerized lab systems includes the PC controlling your qualified instruments. This is an integral audit point that must be maintained to ensure compliance. Ah, but you’re...
Quality & Compliance
How critical is the Technology Transfer phase of new drug development?
During the development phase of a new drug, great pains are taken to characterize the molecule and to run a myriad of laboratory and animal tests to determine the product attributes, toxicology...
Quality & Compliance
Does Your Training Program Have Traction?
With the spotlight these days on Data Integrity, it may be easy to lose sight of some fundamental Quality Systems. Core Quality Systems include Document Management, Investigation Management, and...
Regulatory Sciences
FDA Addresses Extrapolating Adult Data for Pediatric Use
In 2004, the FDA issued a guidance document, entitled “Premarket Assessment of Pediatric Medical Devices.” The guidance stated that, when consistent with scientific principles, data can be...
Quality & Compliance
CPV versus APR, What’s the Difference?
As an outcome of the 2011 Food and Drug Administration Process Validation Guidance, there has been ever increasing interest in the pharmaceutical industry to establish formal Continued Process...