Regulatory Sciences
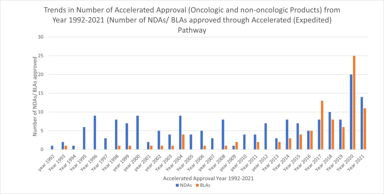
FDA Accelerated Approval Pathway: A Potential Missed Opportunity for Sponsors
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...
Regulatory Sciences
What You Need to Know About Developing Vaccines
An unlikely beacon of hope from the otherwise disastrous Covid pandemic, may come in the form of renewed attention towards approaches to vaccine development. The Importance of Vaccines The...
%20VII%20and%20Type%20D%20Meetings-%20A%20New%20Mechanism%20for%20Interacting%20with%20FDA.jpeg?width=384&height=256&name=Prescription%20Drug%20User%20Fee%20Act%20(PDUFA)%20VII%20and%20Type%20D%20Meetings-%20A%20New%20Mechanism%20for%20Interacting%20with%20FDA.jpeg)
Prescription Drug User Fee Act (PDUFA) VII and Type D Meetings: A New Mechanism for Interacting with FDA
For those who have been awaiting Congressional reauthorization of PDUFA, the wait is over. On September 30, 2022, the President signed into law the FDA User Fee Reauthorization Act of 2022. We...

What You Need to Know About CBER Pre-IND Meetings
The FDA provides several opportunities to hold meetings with Sponsors to gain clarification and agreement on the development of medicinal products. At the preliminary stages of development, one such...
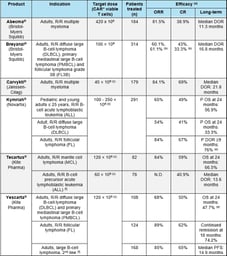
CAR-T Cells: Challenges, Lessons Learned, and Guidance for the Clinical Development
It comes as no surprise to any pharmaceutical or biotech company that planning the clinical development of CAR-T cells is an extremely challenging endeavor: high efficacy is expected in each targeted...

Regulatory Sciences
How to Comply with the Nitrosamine Regulations for Your New Drug Product Marketing Applications
Are you in the development phase for your medicinal product? Have you assessed your manufacturing processes with respect to the requirements for investigating the potential presence of nitrosamine...
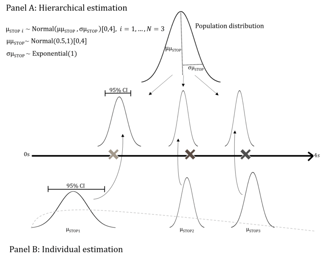
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
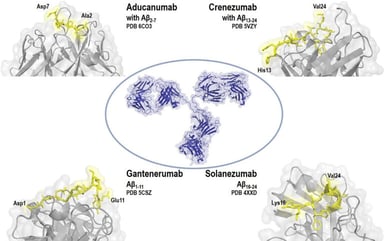
Regulatory Sciences
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Regulatory Sciences
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...

Regulatory Sciences
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA), FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...
Regulatory Sciences
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement
Pharmaceutical companies should understand EU Health Technology Assessment (HTA) authorities requirements early in the product development phase. Engagement with HTA authorities during clinical...
Regulatory Sciences
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

