
Regulatory Sciences
Navigating the German Pharmaceutical Landscape: Part One
Regulatory Considerations and Market Entry Germany is home to one of the most advanced healthcare systems in the world, making it an attractive but complex market for pharmaceutical companies. A...

Quality & Compliance
Building Clinical Quality Assurance: Unlock the Full Potential of Your Clinical Development
In the dynamic world of pharmaceuticals and biotechnology, small Sponsor companies are often the unsung heroes driving innovative therapies through the complex journey from concept to clinic. With...

Quality & Compliance
Ensuring Quality in Clinical-Phase Drug Manufacturing: A Comprehensive Guide
Importance of Quality Assurance in Clinical-Phase Drug Manufacturing Quality Assurance (QA) is a critical function in the pharmaceutical industry, ensuring that every aspect of drug manufacturing...

Demystifying CAPA Management: Overcoming Challenges in the Fast-Paced World of GMP
This article has been updated since its original publication date. Navigating the complexities of Corrective Action / Preventive Action (CAPA) in the drug and medical device industries often poses a...

Quality & Compliance
How to Give Quality Assurance the Respect It Deserves During a Post-Merger Integration
A typical post-merger integration (PMI) between two companies is challenging, time-consuming, stressful, and resource intensive. Even the companies most experienced with integration find these...
Quality & Compliance
What is Software Quality Assurance (SQA) and Do You Need it For Your Business?
If your only method of quality assurance is software testing, you're wasting valuable time and resources. Software Quality Assurance (SQA) is integrated into your software development life cycle to...
Quality & Compliance
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...
Quality & Compliance
Does Your Training Program Have Traction?
With the spotlight these days on Data Integrity, it may be easy to lose sight of some fundamental Quality Systems. Core Quality Systems include Document Management, Investigation Management, and...
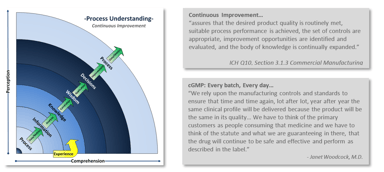
Quality & Compliance
Does your Quality System stand up to the challenge?
Implementing and maintaining a Quality System is a complex challenge. It is as much as an art as it is a science. A company’s Quality System establishes the framework to manage and maintain...
Gravity and Quality Assurance
As we’ve now moved on from another cold winter month, I was captivated recently thinking about Quality Assurance and our collective responsibilities. All kinds of things come to mind; Deviations,...

Quality & Compliance
CAPA and the Importance of Effective Technical Writing
My apologies to you in the technical writing profession out there, but there are not too many topics that are as bland and unappealing as technical writing. Personally, I'd rather step on a sharp...
Sneak Preview: What Will the Quality Metrics Initiative Look Like?
I had lunch in a restaurant the other day. During my meal, I noticed that there weren't many other customers. I didn't give it much thought until I walked out and read the restaurant's hygiene grade...
Who Owns Quality?
When it comes to the Healthcare industry (e.g. pharmaceuticals, medical devices, dietary supplements, etc.) who owns a company's Quality Assurance practices? Without a doubt the Quality organization...
Why Measure and Report Quality Metrics?
A wise man recently told me that “honor and respect should be given freely, but trust is earned”. Whether it’s in politics, friendships, church, marriages, parenting or even the business world,...
Why is CAPA so Important Anyway?
Corrective and preventive action (CAPA) can be viewed differently by employees within pharmaceuticals and medical device companies. Some see CAPAs as simply an onerous task given to them to complete...