
Regulatory Sciences
Navigating the New EU Health Technology Assessment Regulation (HTAR): Are You Ready?
The HTAR in the EU has come into force – what Health Technology Developers (HTDs) need to know Getting your health technology product to the market is complex and usually consists of several steps...

Medical Information
German Pharmaceutical Compliance: The Critical Role of Medical Information Teams
When a pharmaceutical company completes clinical trials and prepares to launch a medical product, the focus often shifts to regulatory approvals, market access, and commercialization. However, one...

Regulatory Sciences
Leveraging Single-Arm Trials for Regulatory Approval: Insights from EMA's Reflection Paper
The pharmaceutical industry has shown a growing interest in single-arm trials due to their potential to expedite drug development. However, several challenges and concerns remain. The European...

Regulatory Sciences
European Pharma Regulations: 2024 Review and 2025 Trends to Watch
Gaining a Competitive Edge: 2024's European Pharma Regulatory Review & Trends to Look for in 2025 and Beyond As the pharmaceutical landscape in Europe evolves rapidly, regulatory frameworks are...

Regulatory Sciences
Navigating Market Access & Reimbursement Strategy for Medical Devices in Europe
Background Developing medical devices (MDs), including in vitro diagnostic medical devices (IVDs), requires careful and result-oriented planning throughout the development process. One critical...

Pharmacovigilance
EU Clinical Trials Regulation: Time Is Running Out, Are You Ready?
On January 31, 2022, the EU Clinical Trials Regulation 536/2014 (CTR) came into force with a transition period of three years. Now, as that transition period comes to a close, the process and need...

Quality & Compliance
Navigating the Nordic Pharmaceutical Landscape: Key Considerations for a Successful Launch
The Nordic region, renowned for its world-class healthcare, presents unique opportunities and challenges for pharmaceutical companies. Understanding the Nordic pharmaceutical landscape is crucial for...

Regulatory Sciences
How to Create Successful Pricing and Reimbursement of your Pharmaceutical Product in the Nordics
Our Nordic region features world-class healthcare. Understanding the Nordic healthcare and market access landscape is crucial for successful pricing and reimbursement of your pharmaceutical product....

Regulatory Sciences
The Moving Regulatory Landscape for Gene Therapy Trials in EU: Part 2
Change in the Submission of the Summary Notification Information Format The Summary Notification Information Format Form As of January 31, 2023, Sponsors are required to submit a Clinical Trial...

Quality & Compliance
The Moving Regulatory Landscape for Gene Therapy Trials in EU: Part 1
The Benefit of the Common Application Form for GMO Applications GMO Application Not Included in Clinical Trial Regulation With the transition period for the Clinical Trial Regulation No 536/2014...
Regulatory Sciences
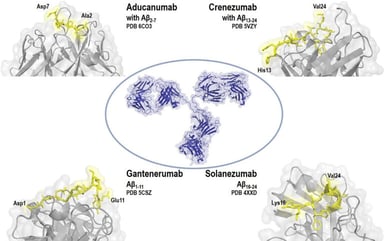
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Regulatory Sciences
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...
Regulatory Sciences
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement
Pharmaceutical companies should understand EU Health Technology Assessment (HTA) authorities requirements early in the product development phase. Engagement with HTA authorities during clinical...

Regulatory Sciences
UK Paediatric Investigational Plans – what do you need to know?? …and how is it all working in practice??
If a marketing authorisation is planned to be submitted in England, Scotland, and Wales (GB), an MHRA-approved paediatric investigational plan (PIP) is required. Up until January 1, 2021, PIPs were...

EMA: Big data use for public health: publication of Big Data Steering Group workplan 2022-25
News July 28, 2022 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its third workplan that sets key actions to be delivered between 2022–25. The new...
How to Fast-Track medicine approval in the UK with the MHRA’s Innovative Licensing and Access Pathway (ILAP)
What is ILAP? What benefits does ILAP provide? How do you access it? With the dust of Brexit settling, the question on most people’s lips (well, those of us in the healthcare sector anyway!) was:...