%20inspection%20procedures.jpeg?width=384&height=256&name=EMA%20Good%20clinical%20practice%20(GCP)%20inspection%20procedures.jpeg)
EMA Good clinical practice (GCP) inspection procedures
The Good Clinical Practice (GCP) Inspectors Working Group has developed procedures for the coordination, preparation, conduct and reporting of GCP inspections requested by the European Medicines...
Regulatory Sciences
Global regulators agree on key principles on adapting vaccines to tackle virus variants
On 30 June, regulators from around the world discussed emerging evidence to support adaptation of COVID-19 vaccines as the SARS-COV-2 virus continues to evolve during a workshop co-chaired by the...
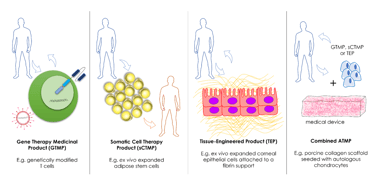
How to Leverage EMA’s ATMP Classification
Advanced therapy medicinal products (ATMPs) have emerged as ground-breaking therapies for rare diseases and other conditions with unmet clinical needs. As of 2022, sixteen ATMPs have been approved by...
Maximising on Scientific Advice Procedures in Europe
A unique opportunity to interact with medicine regulators in Europe Are you considering requesting scientific advice in Europe? We can help you navigate the various procedures within the European...