Unlocking Success with Clinical Trial Safety Monitoring During a Pandemic
Earlier this year I wrote to you about US FDA March 2020 issuance of a new guidance for industry, Investigators, and Institutional Review Boards regarding the conduct of clinical trials during the...

Meet the Expert: Matthew Weinberg
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Regulatory Sciences
Why Drug Approvals Are Never Slam Dunks
It's the FDA, Not the NBA Aside from being a spectacular thing to watch, the slam dunk is the highest percentage shot a basketball player can take. The likelihood of getting the ball in the basket is...
Clinical Research Solutions
The Evolution of Medical Writing
Medical writers must not only have solid writing skills, but knowledge in the context and terminology of medical topics. At times, they may even need to analyze the logic of a passage. Examples of...
Regulatory Sciences
Filing Using Registry-Based Studies? EMA Issues Draft Guidance
Recruiting clinical studies in a niche disease area can be challenging, but disease registries can provide the solution. The European Medicines Agency (EMA) has issued draft guidance on how...

Quality & Compliance
Uncover Opportunities for Improvement with an Annual Product Review
The Annual Product Review (APR), also known as the Annual Product Quality Review (APQR), is required for marketed products in an FDA-regulated environment. You may ask, "Why would I want to perform...
Pharmacovigilance
Is Your Pharmacovigilance Team Ready for Brexit?
As we approach the final months of 2020, the pharmaceutical world begins, once again, to focus its thoughts on the impact of Brexit, not least in the world of pharmacovigilance. Of course, the UK has...
Quality & Compliance
EudraLex Volume 4, Annex 1 Update: What You Need to Know
EudraLex Volume 4, Annex 1 provides guidance for the manufacturing of sterile medicinal products that are intended for the European market. It has been updated several times, with the latest revision...
Clinical Research Solutions
5 Ways the Medical Industry is Using Data Science
In the era of technological disruption, data science is a disruptor for the books. Today’s data scientists develop processes, algorithms, and systems to mine structured and unstructured data with the...
Clinical Research Solutions
10 Things You Need to Know about FDA’s Final Rule on Importing Prescription Drugs from Canada
Last week, FDA issued a final rule regarding the importation of certain prescription drugs from Canada. This action was taken by the agency as part of the Safe Important Action Plan, and was done in...
How to Prepare for Laboratory Partner Selection during CBD Product Development
The interest in developing consumer products or therapies derived from Cannabis or CBD is continuously growing. As these new products come to market, there is increasing need to comply with...
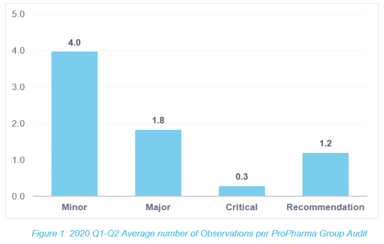
Quality & Compliance
How Many Observations are Hiding in Your Quality System?
Reflecting on 2020, we have become accustomed to the global shift into a world of virtual, remote, restricted, and paused. One no longer flinches when hearing that something has been modified,...
Clinical Research Solutions
6 Tips to Prepare Your Medical Cannabis Facility for Inspection
You may be considering building a new facility for growing, harvesting, and processing medical cannabis, or perhaps you have an existing facility and want to export to the European Union. What should...
3 Basic Data Integrity Principles to Protect Value and Drive Success for Cell and Gene Therapy/ATMP Development
Cell and Gene Therapy (CGT)/Advanced Therapy Medicinal Products (ATMPs) have the incredible potential to cure devastating illnesses, such as cancer, on a more personalized level. But, due to the...
Regulatory Strategy in Pharma & Biotech Submissions Trial Design
Drug development can be a protracted and multifaceted process. This is often the case for startups and newer organizations, which may not have dedicated regulatory compliance departments and...






