July 9, 2014
July 9, 2014

In case you haven’t noticed, FDA is tired of being the “bad guy”. While they do not back-off of their responsibility to enforce the applicable Code of Federal Regulations for a millisecond, their budgets are strained, they are frequently understaffed and, human nature being what it is, are weary of playing the never-ending game of “Gotcha” with the companies they regulate.
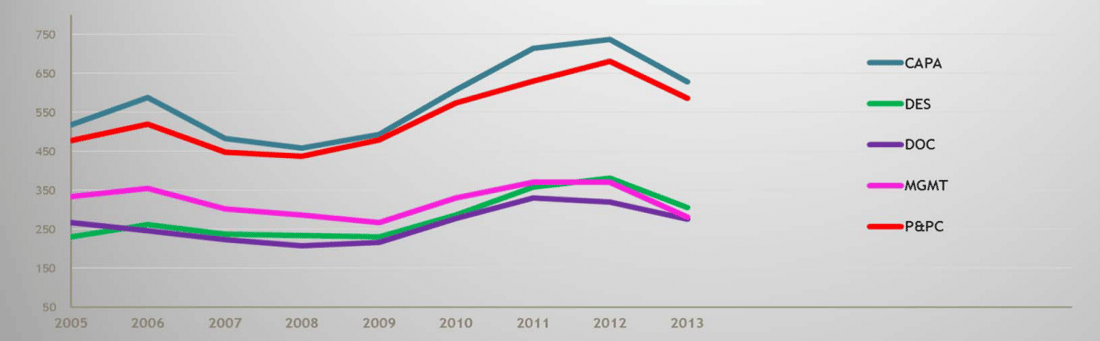
FDA is also populated with some very sharp and talented people, and they know how to analyze data. For example, FDA is fully aware that the chart showing the categorized reasons for medical device 483s in 2005 is essentially the same today. That’s right, the same 483-worthy problems (CAPA, Design Control, Documentation, Production and Process Control, i.e., Process Validation, and Management Responsibility) that have existed in the device industry for years have not shown any appreciable improvement, despite the implementation of the Quality System Regulation circa 1997.
2005-2013 FDA Form 483 Observations: 2005 – 20013; Medical Devices
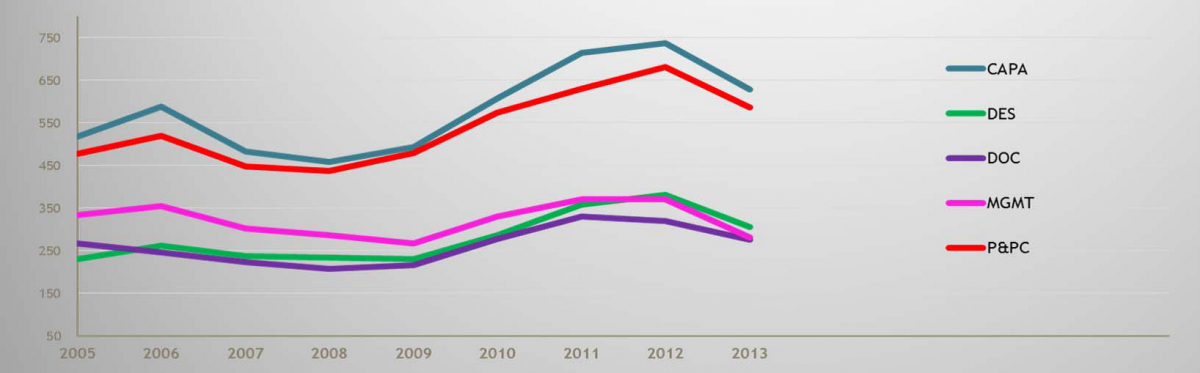
Remember the definition of insanity where one does the same thing time after time and expects a different result? Add the earlier "tired of being the bad guy" comment and we have a FDA willing to try new approaches to old problems.
There are recent initiatives underfoot that FDA hopes will create FDA-industry partnerships, increase transparency, utilize data more effectively, and have a positive impact on industry’s bottom line. I will explain these initiatives in a forthcoming blog, but will use this time and space to explain one of the initiative’s fundamental drivers, that being the difference between Compliance and Quality.
Compliance is simply putting out sufficient effort to meet cGMP’s minimum requirements and measuring compliance against the law. Quality, on the other hand, is derived from a systematic continuous improvement process whereby management, resources, products, and measurement systems are aligned and operated in such a way that continuous improvement and compliance is an inescapable outcome. An organization will never achieve those benefits with a compliance-only approach.
In an upcoming blog post, I’ll be discussing how these differences affect productivity and profitability.
Learn more about ProPharma's Compliance services. Contact us to get in touch with our subject matter experts for a customized Compliance presentation.
TAGS: Compliance Quality & Compliance Life Science Consulting
July 15, 2015
Business sustainability is an often heard buzz phrase, but how does it apply when a costly process validation is imminent? Process validation is not only a regulatory compliance issue; it is also a...

May 11, 2016
Implementing and maintaining a Quality System is a complex challenge. It is as much as an art as it is a science. A company’s Quality System establishes the framework to manage and maintain...
September 2, 2014
In June 2014, the FDA issued two draft social media guidance documents that may not clearly answer all of the questions that the drug and device industries have about how to use social media to...