
Quality & Compliance
Annex 11 2011 Version vs. Annex 11 2025 Draft Version: What are the Differences and Enhancements?
The GMP/GDP Inspectors Working Group and the PIC/S Committee jointly recommended that the current version of Annex 11 on Computerised Systems be revised to reflect changes in regulatory and...
Implementing eQMS in a Regulated Environment
Everything we do today is traceable. The integrity of the data, documents and decisions needs to be defendable and consistent. Electronic Quality Management Systems (eQMS) are an effective way to...
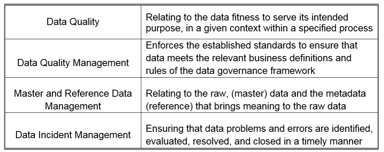
Just How Mature Is Your Data Lifecycle / Data Management Function?
The attention of regulatory agencies continues to focus on data integrity, as observed by the increase of FDA observations over the course of the last few years. Having a proper data lifecycle / data...

Quality & Compliance
Summary Considerations: Use of Electronic Records and Electronic Signatures in Clinical Investigations Under 21 CFR Part 11 – Questions and Answers
Here I provide some key summaries and considerations relative to FDA’s draft guidance that was submitted for review and comment in June 2017. If you don’t want to review the entire guidance, here are...

Quality & Compliance
Data Integrity 101: Is your data compliant?
Data integrity has recently been in the agency spotlight. In part because of the draft data integrity guidance issued April 2016, but primarily due to an increased number of inspection findings...

Quality & Compliance
Is Your Laboratory PC Cloned From the Proper Image?
Managing compliance for computerized lab systems includes the PC controlling your qualified instruments. This is an integral audit point that must be maintained to ensure compliance. Ah, but you’re...
Quality & Compliance
Top Takeaways from FDA's Guidance on Data Integrity
For those of you unaware, the FDA submitted a draft guidance on data integrity for comment in April. This is significant in that the last formal written guidance provided from FDA on this subject...
Everything you to need to know about Audit Trails
In today’s validated lab environment, knowing the importance of an audit trail in computerized laboratory systems is just one of the integral qualification tasks that the ProPharma’s Computer System...
Quality & Compliance
CSV Considerations Around Data Integrity
Data integrity is a current hot topic, but not a new one, within the life sciences industries and associated product supply chains. This article will not delve into why there have been so many recent...
Looking to the Cloud for your Business
computer susteIt’s a Friday afternoon. Quarter’s end. Your V.P. of Regulatory Affairs calls your office bellowing something about not being able to process the latest submission data- can’t access...

Quality & Compliance
Lean CSV Requirements and Planning – How to Reduce Waste, Increase Value, and Maintain Compliance
The diagram below depicts an example CSV lifecycle alignment with ASTM E2500 and GAMP. Building onto a previous post, Lean CSV - How to Reduce Waste and Increase Value, today's blog focuses on...

Improving Computerized System Quality Through Design Verification
Unverified Design – An Example For those of us who travel routinely, one of the most sought-after treasures in the typical airport terminal is an electrical outlet. With our dependency on mobile...
Computer System Validation: Resolutions for the New Year
How are you doing with those New Years’ resolutions? Whether or not you’re a resolution maker (or breaker), you can use this time of year to take stock of where things stand, including your validated...
Quality & Compliance
Lean CSV – How to Reduce Waste and Increase Value
What is lean? Lean is a business system focused on continuously improving processes by reducing the time taken and the waste involved in delivering increasing value to the customer. Customers in this...
Quality & Compliance
Risk-Based Computer System Validation and Rational Testing
Risk based approaches to validation of computerized systems have been heavily promoted since the publication of GAMP 5 and ASTM E2500. Yet we continue to see examples of validation overkill in the...
