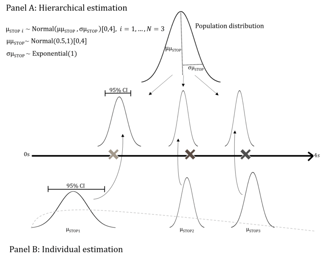
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
August 19, 2022 EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is...

FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA) , FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...
EMA COVID-19 guidance: research and development
The European Medicines Agency (EMA) provides support to medicine developers researching and developing potential COVID-19 medicines. Dedicated guidance, rapid procedures and contact points are...
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

FDA Recognizes August as National Immunization Awareness Month
National Immunization Awareness Month provides us an opportunity to think about how far the development and advancement of immunization science has come, and its impact on public health. The U.S....

