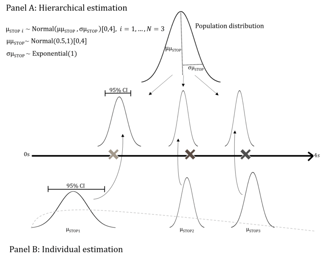
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
Regulatory Sciences
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...

Regulatory Sciences
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA), FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...
Regulatory Sciences
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

Regulatory Sciences
FDA Recognizes August as National Immunization Awareness Month
National Immunization Awareness Month provides us an opportunity to think about how far the development and advancement of immunization science has come, and its impact on public health. The U.S....

Regulatory Sciences
FDA publishes product-specific guidances to facilitate generic drug development
Today, FDA published a new batch of product-specific guidances (PSGs). PSGs provide recommendations for developing generic drugs and generating the evidence needed to support abbreviated new drug...

EMA: Big data use for public health: publication of Big Data Steering Group workplan 2022-25
News July 28, 2022 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its third workplan that sets key actions to be delivered between 2022–25. The new...
Regulatory Sciences
Global regulators agree on key principles on adapting vaccines to tackle virus variants
On 30 June, regulators from around the world discussed emerging evidence to support adaptation of COVID-19 vaccines as the SARS-COV-2 virus continues to evolve during a workshop co-chaired by the...
EMA adopts first list of critical medicines for COVID-19
News On 7 June 2022, EMA's Medicines Shortages Steering Group (MSSG) adopted the list of critical medicines for the COVID-19 public health emergency. The medicines included in the list are authorised...

FDA publishes MAPP 5223.6, Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA
Today, FDA published a new Manual of Policies and Procedures (MAPP), “Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA (5223.6).”...

