
Regulatory Sciences
Tips for Preparing Successful FDA 510(k) Submissions
Securing FDA clearance through the 510(k) process is a critical milestone for many medical device manufacturers seeking market entry in the US. While the pathway is well-defined, many submissions...
Quality & Compliance
Creating an Effective Global Audit Strategy for Medical Device and Diagnostic Companies: A Tale of Two Paths
The Crossroads of Global Compliance For medical device and diagnostic companies operating in today’s global marketplace, success depends on more than just innovative products — it hinges on a...

Quality & Compliance
FDA Inspection Readiness: Top Observations and How to Avoid a Form 483
For medical device manufacturers, FDA inspections are a critical component of regulatory oversight and quality assurance. Whether part of a routine surveillance program, pre-approval inspection, or...

Quality & Compliance
FMEA Is Not a Crystal Ball: Why You're Missing Critical Risks in Medical Device Lifecycle Management
Imagine trying to predict how a medical device could cause harm—but you're only allowed to look at one failure at a time, with no context, interactions, or real-world complexity. It's like peering...

Regulatory Sciences
Navigating FDA's Q-Submission Process: A Strategic Advantage for Medical Device Developers
In the highly regulated medical device industry, navigating FDA's submission process can be daunting, especially for start-ups. However, FDA's Q-Submission (Q-Sub) program offers a valuable...

Regulatory Sciences
Navigating a Shifting Regulatory Landscape: The Impact of FDA Layoffs on Medical Device Developers
On Thursday, March 27, 2025, HHS announced that they would be conducting a reduction in force impacting nearly 3,500 full time FDA employees – nearly 20% of the Agency’s entire workforce. This comes...

Regulatory Sciences
Leveraging Single-Arm Trials for Regulatory Approval: Insights from EMA's Reflection Paper
The pharmaceutical industry has shown a growing interest in single-arm trials due to their potential to expedite drug development. However, several challenges and concerns remain. The European...

Regulatory Sciences
Navigating Market Access & Reimbursement Strategy for Medical Devices in Europe
Background Developing medical devices (MDs), including in vitro diagnostic medical devices (IVDs), requires careful and result-oriented planning throughout the development process. One critical...

Clinical Research Solutions
Navigating the Regulatory Maze: Clinical Data for Medical Device Approval
In the world of medical devices, particularly those categorized as moderate to high-risk, clinical data is a critical component of the regulatory approval process. This data, presented to regulatory...

Regulatory Sciences
FDA eSTAR Template: Navigating FDA's 510(k) Submission Requirements
Implementing the eSTAR Format The eSTAR template is a positive step for both CDRH and medical device Sponsors; but, as with any new tool, there are challenges to utilizing the template. Sponsors need...
FDA Finalizes the Breakthrough Devices Program Guidance and Reinforces Innovative Medical Device Manufacturers that Help to Address Health Inequities
The FDA's Breakthrough Devices Program is intended to expedite the development of innovative technologies for patients with life-threatening or irreversibly debilitating diseases or conditions. The...
Regulatory Sciences
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...
Quality & Compliance
Roadmap for Successful IVDR Transition, Part II: Technical Documentation & Software
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching (May 2022). In this blog series, we discuss the final months before the IVDR date of application along with...
Quality & Compliance
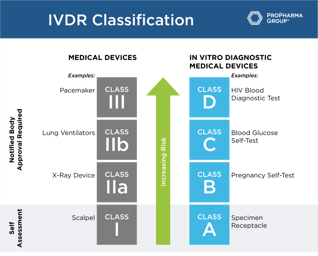
Roadmap for Successful IVDR Transition
Roadmap for Successful IVDR Transition: The compliance dates for the In Vitro Diagnostics Regulation (IVDR) will become effective on May 26, 2022. To help you with the IVDD to IVDR transition, we've...
Regulatory Sciences
Understanding The Medical Device Single Audit Program
The Medical Device Single Audit Program (MDSAP), initiated by the International Medical Device Regulators Forum (IMDRF), created a global approach to auditing and monitoring the manufacturing of...
Regulatory Sciences
The Human Factor - Preparing Your Device for Usability Testing
When it comes to usability studies, the focus should be on effectively preparing the medical device for use in humans. Whether a Sponsor is conducting formative testing or validation testing, the...