Quality & Compliance
Should Data Integrity Detection be a Part of Routine cGMP Training Programs?
The FDA’s focus on data integrity in recent years has proven that it remains an industry issue. The focus has resulted in significantly increased issuance rates of 483 observations, warning letters,...
Quality & Compliance
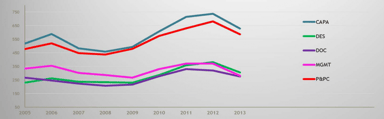
FDA’s Most Frequent 483 Observations for 2016: A Reflection of Industry’s Compliance
At the beginning of each year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center. Image Source: FDA I find that reviewing these metrics provides a...
Quality & Compliance
Understanding the 21st Century Cures Act: Part II
The 21st Century Cures Act, (Passed December 13, 2016), represents three years of cooperation between Congress, the FDA, and industry to modernize the current legal framework within which the FDA...
Quality & Compliance
Understanding the 21st Century Cures Act: Part I
The recent passage of the 21st Century Cures Act (passed December 13, 2016) marks a significant milestone for medical device and drug development. I recently attended a meeting held by the Food and...
The Investigation Best Practices to Avoid FDA 483 Observations
For a number of years, discrepancy and failure investigations within the pharmaceutical industry have been populating the Top 3 of a Food and Drug Administration (FDA) Observation list....
Problem Solving: What’s the Best Approach?
Those of you in the pharmaceutical, biotech and medical device industries who encounter process and product problems on a regular basis, you likely grimace when one lands in your lap. There is a...
Quality & Compliance
Quick Guide: cGMP for Phase 1 Investigational Drugs
As Phase I clinical trials mark the first time that an investigational new drug is administered to humans, these studies are subject to appropriate current Good Manufacturing Practices (cGMP) in...
Quality & Compliance
Value Found in FDA Warning Letter Reviews
Starting with the issuance of a 483, the stepwise FDA enforcement process can be illustrated as follows: Given the seriousness of a Seizure and Injunction scenario, not to mention potential jail time...
Quality & Compliance
FDA Inspection? Rely on Your Vendors
If you’ve spent a reasonable amount of time in an FDA-regulated industry, you’ve most likely been part of an FDA inspection. Should you be more fortunate, you have participated directly with the...
Regulatory Sciences
FDA Issues Final Guidance on Providing Regulatory Submission in Electronic Format – The eCTD Mandate is Coming
On May 5, 2015, the Food and Drug Administration (FDA) released the final guidance on Providing Regulatory Submissions in electronic format. The guidance implements the electronic submission...
Is social media the next monitoring emphasis for the OIG?
In June 2014, the FDA issued two draft social media guidance documents that may not clearly answer all of the questions that the drug and device industries have about how to use social media to...
Quality & Compliance
The Difference Between Quality and Compliance, Part II
In our previous blog, "The Difference between Quality and Compliance", I mentioned that there are recent initiatives underfoot that FDA hopes will create FDA-industry partnerships, increase...
Quality & Compliance
The Difference Between Quality and Compliance
In case you haven’t noticed, FDA is tired of being the “bad guy”. While they do not back-off of their responsibility to enforce the applicable Code of Federal Regulations for a millisecond, their...
Risk Assessments Mitigate Risk for Bigger and Smaller Companies Alike
Earlier this year, the Officer of Inspector General (OIG) put smaller life sciences companies on notice that they should put in place a risk assessment process as part of their corporate compliance...
Quality & Compliance
Manage Speaker Program Risk More Effectively with a Needs Assessment
Over the past several years, pharmaceutical company Corporate Integrity Agreements (CIAs) have routinely required needs assessments in connection with the engagement of healthcare professionals...
Quality & Compliance
Evaluating the Effectiveness of a Corporate Compliance Program: How Does Your Program Measure Up?
At the recent Pharmaceutical Compliance Congress (PCC), Zane Memeger, U.S. Attorney for the Eastern District of Pennsylvania stated that one of the key questions that a company should ask about its...