In our previous blog, "The Difference between Quality and Compliance", I mentioned that there are recent initiatives underfoot that FDA hopes will create FDA-industry partnerships, increase transparency, utilize data more effectively, and have a positive impact on industry’s bottom line. As I mentioned, one of the initiative’s fundamental drivers is the difference between Compliance and Quality.
Compliance vs. Quality: Understanding the Core Difference
Compliance is simply putting out sufficient effort to meet cGMP’s minimum requirements and measuring compliance against the law. Quality, on the other hand, is derived from a systematic continuous improvement process whereby management, resources, products, and measurement systems are aligned and operated in such a way that continuous improvement and compliance is an inescapable outcome. An organization will never achieve those benefits with a compliance-only approach.
Continuous Improvement (CI) holds the key to not only compliance, but even more so to improved productivity and therefore increased profitability (why regulated companies view compliance/quality as a burden and not an opportunity to capitalize upon is a mystery).
The ISO13485 Quality Management System model is a good example of a CI process.
A disciplined approach leads to a well-run and efficient system that will always identify areas and opportunities in need of improvement. Over time, and as the wheel starts to accelerate, each opportunity for improvement is capitalized upon. With multiple problems come multiple opportunities for improvement. Assuming that corrective and preventive actions deployed are truly effective (the wheel is spinning faster now), there is no other outcome than a system that constantly reduces waste (thereby increasing productivity and profitability), drives process knowledge (thereby increasing process control and reducing variability), and creates a quality system-culture based on quality metrics and data.
In the context just mentioned, Quality System-driven business benefits are easier to talk about and realize than they are when the discussion is restricted to a strictly cGMP compliance focus. Let’s say that an organization has created a CAPA to address a severe and frequent process failure, and that this process as designed relies on end-of-process QC testing. Effective root-cause analysis and problem solving techniques are used to better understand and control the process, giving way to the monitoring of critical process parameters that allow the organization to abort or correct the process earlier than was previously possible (well before QC testing requirements). The organization has now reduced overall waste by correcting a process in mid-stream rather than waiting for QC testing and re-working the product (or aborting the process and not wasting time and money to complete it only to have the product rejected by QC because it fails to meet specifications).
The following diagram illustrates the notion of what reduced waste can mean to an organization’s bottom line:
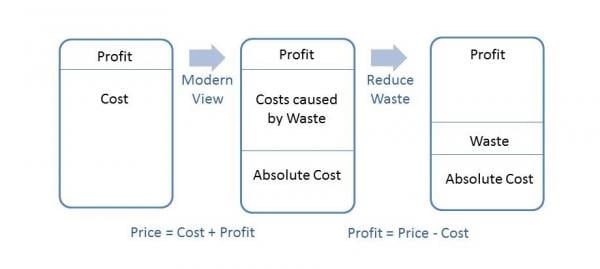
The traditional view of profitability used to be simplistic. An organization had costs to manufacture and release a product. Slap a profit margin on top of their costs and that was the price to the customer; however, taking a closer look at costs; those that are fixed and absolutely necessary (payroll, facilities, utilities, etc.) and those caused by waste such as in the previous example, reveals an opportunity. In that example, the organization had fixed their process and therefore did not spend dollars manufacturing a defective product as they had pre-fix. Those unspent dollars contribute directly to the bottom line as illustrated above. Reduction of waste increases profitability in this manner.
The development of a Quality System that continually improves itself is not a new concept, but does represent FDA’s current thinking and underlies the initiatives now taking hold and gaining momentum. I’ll discuss these further in depth in a future blog.
Learn more about ProPharma'sComplianceservices.
Contact us to get in touch with our subject matter experts for a customized Compliance presentation.


