Clinical Research Solutions
Changes to the Animal Welfare Act Affecting Animal Research Facilities
Does your organization conduct or outsource testing to an Animal Research Facility? If so, are you aware of the changes that have been implemented to the AWA (Animal Welfare Act) by the Animal and...

7 Critical Factors for Successful Selection of CDMO for Cell and Gene Therapy Manufacturing
Developing, optimizing, and manufacturing Advanced Therapy Medicinal Products (ATMP's), such as Cell and Gene Therapy (CAGT) products is extremely complex. The choice of a reliable Contract...

Project Management isn’t brain surgery, but why is it so hard?
Here is the scenario: you are the leader of a group and you have been given the responsibility of driving the completion of a new initiative, an early or late-stage drug or devices program, or a...
Annex 21: What You Need to Know
It’s finally here, a guidance on how to handle medicinal products imported from outside EU/EEA. If you’ve ever wondered what the expectations are on these imported medicinal products, you’re in for a...
CMC Regulatory Dossier Compliance: A GMP Requirement
Maintaining compliance in the dynamic regulatory Chemistry, Manufacturing and Controls (CMC field can be quite a challenge. A CMC regulatory dossier compliance assessment is a critical component and...
Decentralized Clinical Trials: Changing the Landscape of Clinical Trials
One essential consideration in clinical trial development is whether the trial will represent a given population. The most representative trials use recruitment techniques and advanced statistical...
How to Implement an Effective Audit Trail
Maintaining an audit trail is a regulatory compliance requirement, but what makes an audit trail beneficial for maintaining effectiveness and complying with regulations? This blog will explain what...
How to Ensure Your Workforce is GMP Compliant
As the world tries to get back to some semblance of normalcy after the coronavirus pandemic, pharmaceutical, biotech and med device companies are anticipating the day when the FDA returns to normal...
How to Prepare for the Three Type B Meetings with the FDA
The Food and Drug Administration (FDA) has laid out a drug development continuum that includes three milestones, or Type B meetings. Earliest is the Pre-IND meeting, the second is the End of Phase 2...
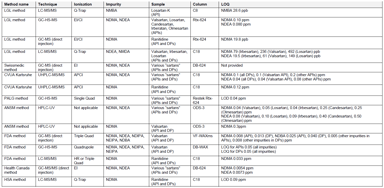
Analytical Methods for the Investigation of Carcinogenic Nitrosamines in APIs and Drug Products
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...
2020 in Review: Reflecting on the Biggest Events & Breakthroughs
2020 has been an unusual year. You could also describe this year as different, difficult, and challenging, just to list a few – the list could go on for days. And yet, this year leaves us with...
3 Basic Data Integrity Principles to Protect Value and Drive Success for Cell and Gene Therapy/ATMP Development
Cell and Gene Therapy (CGT)/Advanced Therapy Medicinal Products (ATMPs) have the incredible potential to cure devastating illnesses, such as cancer, on a more personalized level. But, due to the...

Clinical Research Solutions
Pre-Approval Inspection (PAI): What it is and How to Prepare
What is a Pre-Approval Inspection (PAI)? A pre-approval inspection (PAI) is performed to provide the Food and Drug Administration (FDA) assurances that a manufacturing site named in a drug...
Quality & Compliance
How Data Integrity Supports a Smooth Transition to Pharma 4.0
The coming pharmaceutical industrial revolution, Pharma 4.0, is an implementation of new systems into the various manufacturing processes leading to an automated production. Introduction of these...
Clinical Research Solutions
How Coronavirus Brings Forward New Technologies: The Future of Remote CSV
Is remote Computerized Systems Validation the future standard? Crises are fertile ground for inspiration and creativity resulting in practical new ideas for the near future, as is the case during the...
FDA Emergency Use Authorizations 101: COVID-19 Medical Devices
Everything You Need to Know About Emergency Use Authorizations for Medical Devices to Test and Diagnose COVID-19 In early February, the Secretary of HHS declared that the circumstances presented from...