The coming pharmaceutical industrial revolution, Pharma 4.0, is an implementation of new systems into the various manufacturing processes leading to an automated production. Introduction of these systems deliver significant efficiencies for production and have evolved to transform the way manufacturing facilities are designed, supported and protected. Although the stringent regulations do not prohibit the adoption of new practices, the regulations are often the main factor that slows down this transformation. For this reason, the pharmaceutical industry has lagged in transition towards Pharma 4.0 and many manufacturers are fairly far from immersing automated systems into their processes. Here, the Data Integrity (DI) supports a smooth transition to Pharma 4.0 by ensuring that the data generated during these processes is reliable. Only then business owners can make best suitable choices for their organizations, improve quality of their products and contribute to the overall success of the company in the future. Therefore, following DI controls allows drug manufacturers to make a step forward towards integration of automated production systems designed and used in Pharma 4.0.
It is exciting to see that we are now facing the edge of the Fourth Industrial Revolution (Industry 4.0) that requires ingenious integration of new advanced controls in organization and management of Big Data. The Industry 4.0 builds on the developments of the Third Industrial Revolution (Industry 3.0) that introduced computerized production systems and expanded digital networks between them. These networks lead to "cyber-physical” production systems and, therefore, smart factories in which components and people communicate, allowing nearly autonomous production. This also enables communication with other facilities and continuous record of the output information into large data sets, called Big Data. Industry 4.0 is fueled by Big Data processing, interconnectivity, collaborative robotics, artificial intelligence, and digitally distributed cloud service-based architectures. Digitalization enables the change across the organization in data handling along the complete value chain networks.
In this relation, DI controls around data during the manufacturing processes become crucial drivers towards the Industry 4.0 in pharmaceutical industry (Pharma 4.0). This is because organizations make decisions based on this data which ultimately defines the effectiveness of business operations and quality of the products. Data represents a major investment for an organization such as operational data, historical data, research data, and intellectual property. In this relation, the DI controls ensure that the data in an organization is complete, trustworthy, consistent, and accurate throughout the lifecycle of the product. When process is defined and reliable data flows are specified across the IT architecture, the facility matures in relation to recorded information. However, due to increasing complexity and amount of the data created, manufacturers are experiencing issues in embracing new technological breakthroughs and introducing appropriate changes in data handling.
One of the challenges is that data is not static throughout product lifecycle. Trustworthiness of the data in your organization is dependent on the weakest link in this chain (Fig. 1). The failure can occur at any step, for instance, while transferring the data to other systems, altering and updating multiple times. Human error, data transfers, viruses, external intrusions, malware, update failures, or damaged hardware are all factors that can compromise your data flow. For the sake of data security and quality, the access to raw data or certain levels of data should be restricted by using authorization structures. These structures allow or deny certain individuals to make changes or to erase data without previous clearance.
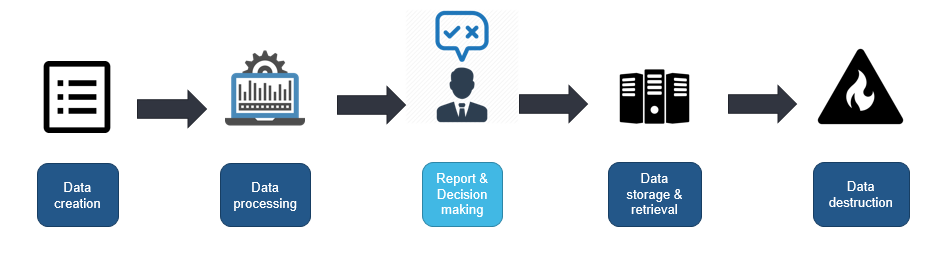
In parallel with the technological progress, authorities respond with additional requirements that ensure continuous improvement of product’s quality. Thus, the attitude of inspectors is changing and, nowadays, greater attention is payed to the controls set in the organization over the data. Here, DI has become one of the most critical points during audits. The effort and resource applied to assure the integrity of the data should be commensurate with the risk and impact of a DI failure to the patient or environment. Essentially, having DI controls in place at the critical process steps will help your organization to smoothly pass audits, avoid poor quality products, recalls, and many other negative consequences.
While data integrity establishes the controls throughout the lifecycle of the product, the sum of arrangements assuring data quality is called data governance. Irrespective of the process, format, or technology in which data is generated, recorded, processed, retained, retrieved, and used, data governance ensures that data is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and the record is available throughout the data lifecycle (ALCOA+). Implementation of these arrangements in your manufacturing processes demonstrate that the data flow and responsibilities are set up correctly.
Setting up DI controls across data lifecycle that are introduced by Pharma 3.0 and demonstrating that generated data is ALCOA will create a robust platform for successful future of your organization. Once DI controls are set up and ALCOA, only then more of the tasks can be delegated to robotics, complementing and gradually substituting the work that humans do, and even perform some tasks beyond this. In addition to this, integration of DI controls in your organization also ensures continuous production, preventing disruptions in the critical process steps during times when human involvement must be limited.
An illustrative example of required limited human involvement in the processes is the COVID-19 pandemic that the world has experienced in 2020. This example should stimulate manufacturers to adapt Pharma 3.0 principles into their manufacturing processes more actively and accelerate transition towards the Pharma 4.0 concept. For successful transition, it is essential that your organization takes an optimized approach from the beginning and integrate new principles and systems correctly with a smart outlook to the future. These first steps and decisions are very important and absolutely necessary in order to meet the advances from Pharma 4.0 and ensure a smooth transition towards automated processes. Not meeting these advances eventually may lead to ineffective or misleading decisions negatively affecting quality of the products and safety of the patients.
In conclusion, the way your organization manages the data lifecycle can significantly influence your business. Mishandling of the data or decisions based on corrupted data can compromise the end user leading to serious consequences for the company’s future such as damaged reputation and loss of profit. To avoid the consequences that may result in poor quality of the products and impair safety of the patients, you can set up the right DI controls over your data now and enter Pharma 4.0 in a natural way.
ProPharma possesses an extensive knowledge and expertise regarding data integrity and can help to facilitate the integration of its controls. To guide you through the implementation of DI principles into your systems and processes, we first assess the maturity level of data management in your organization. Then we identify gaps, prioritize and design a solution boosting one or a number of pre-defined DI areas. Therefore, we are able to provide your company with full support ensuring establishment of robust and optimized controls over your data lifecycle across your organization.
References
- MHRA GxP Data Integrity Definitions and Guidance for Industry, Draft version for consultation, July 2016, MHRA GxP Data Integrity Definitions and Guidance for Industry - Draft version for consultation July 2016
- ISPE GAMP® Guide: Records and Data Integrity (2017)
- WHO Technical Report Series, No. 996, Annex 5: Guidance on Good Data and Record Management Practices, World Health Organization (WHO), 2016, Annex 5 Guidelines on import procedures for medical products.
Authors

Natalia Vtyurina

Emmie Heeren
TAGS: Quality & Compliance Data Integrity Life Science Consulting
