December 4, 2018

December 4, 2018

Over the past 20 years, the traditional approach to drug development has expanded to include the outsourcing of a range of testing and manufacturing functions. As a part of their long-term strategic view, successful Sponsors understand the need to leverage the knowledge and resources that external specialist organizations bring to the table.
Sponsors of every size and type outsource clinical functions to contract research organizations (CROs) and manufacturing functions to contract manufacturing organizations (CMOs).
Virtual companies, run by a small team, outsource functions because they lack the financial or personnel resources it takes to conduct clinical trials and manufacture their drug. Mid-sized companies may outsource so that they can focus on their core competencies while leveraging the niche expertise of contractors. Finally, large companies might decide that it is more efficient for them to outsource functions because doing so will result in greater efficiency, shorter time to market, and lower costs.
While these are all valid business decisions, outsourcing isn’t a means of mitigating risks or transferring compliance obligations to contracted facilities. When it comes to compliance with FDA regulations, the buck stops with the Sponsor. Regardless of the relationship, outsourcing functions doesn’t mean that a Sponsor has outsourced compliance obligations.
Consequently, Sponsors must do everything within their power to ensure that their actions, as well as the actions of the CRO or CMO, are compliant, and it starts with having a quality system in place.
Sponsors are responsible for making sure that every task associated with clinical trials, manufacturing, and distribution of their drug is performed in compliance with FDA regulations. This is achieved by operating with a robust quality system designed to keep the Sponsor and its contracted facilities compliant.
The stronger the Sponsor’s clinical quality system, the more likely their study is to be compliant with Good Clinical Practices (GCPs) and able to quickly progress to the next phase in development. This helps the Sponsor save time and money when working with their CRO to remedy something uncovered by an internal, third-party, or FDA audit.
As for Good Manufacturing Practices (GMPs), a strong quality system provides for the proper design, monitoring, and control of manufacturing processes and facilities. This ensures that drug products meet the FDA’s quality standards.
No matter how well-designed a Sponsor’s quality system is when they are outsourcing functions, it’s crucial that they have a quality agreement that extends the Sponsor’s quality system to the contracted facility.
As a part of a larger outsourcing management plan, a Sponsor should have a comprehensive, written quality agreement with each of their contracted facilities. This agreement is a part of the Sponsor’s quality system and delineates what is expected of both parties by defining the obligations and responsibilities of the quality units of both parties.
Although there is no statutory or regulatory requirement for a quality agreement, regulations do require that quality unit responsibilities and procedures be in writing (21 CFR 211.22(d)). Despite the lack of a legal requirement, in their guidance on the matter, the FDA recommends that Sponsors and contractors operate under a quality agreement. Furthermore, the Agency’s guidance also states that inspectors will seek to review quality agreements during facility inspections.
Again, even though there may be a document that clearly sets out the obligations and responsibilities of the Sponsor and the contracted facility, that document does not have the power to transfer compliance obligations from the Sponsor to the contracted facility. If there is a failure in compliance on behalf of the contracted facility, the Sponsor will always be responsible.
Regardless of the Sponsor’s size or their arrangement with a contracted facility, the Sponsor cannot outsource their compliance obligations. Because of this reality, they must keep their obligations in mind as they work with their contracted facilities.
ProPharma Group is your compliance partner. We work with Sponsors to create a customized quality system design that promotes GCP and GMP compliance while accommodating each Sponsor’s unique circumstances. We start by conducting a gap analysis to determine the strength of their existing quality system and submit a report that details what we found and includes recommendations based on these findings.
Based on a Sponsor’s needs, our team can build out as little or as much of the quality system as requested. When remediation is suggested, we can also help with related project management. From standard operating procedure (SOP) writing to building a quality framework to providing a solid methodology for GCP and GMP audits, our team helps Sponsors mitigate the risk of noncompliance.
Contact us today to talk to our experts about compliance and quality systems.
May 2, 2017
The landscape of clinical trials is evolving. The changes that are happening are due to the increased number of FDA-regulated trials, as well as a rise in the complexity of clinical protocols. As...

October 23, 2023
In today's highly regulated pharmaceutical, biotechnology, and healthcare industries, maintaining compliance with GxP Practices, and other regulatory requirements is paramount. Failure to meet these...
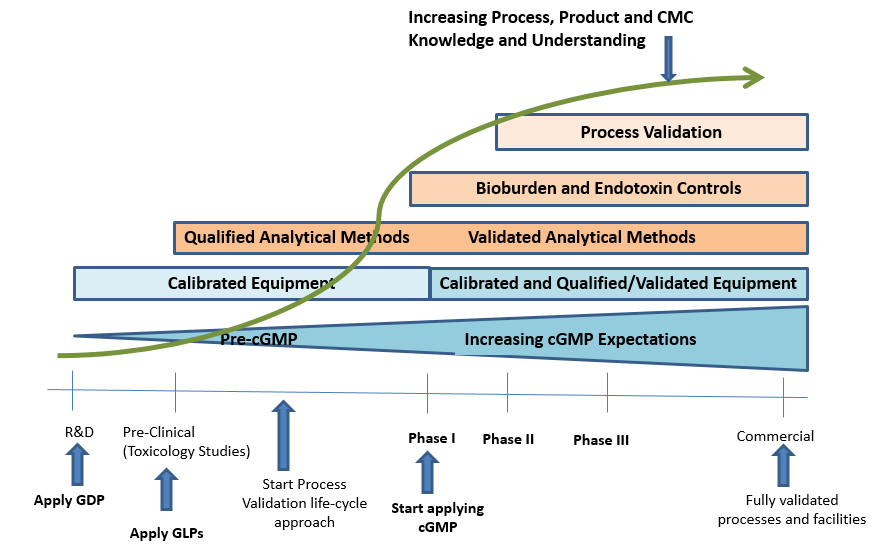
November 23, 2020
Over the past several decades, the traditional approach to drug development and manufacturing has expanded to include the outsourcing of a range of functions from product development and testing, to...