In today's highly regulated pharmaceutical, biotechnology, and healthcare industries, maintaining compliance with GxP Practices, and other regulatory requirements is paramount.
Failure to meet these standards can result in consequences, including regulatory sanctions, product recalls, and damage to a company's reputation.
Compliance auditing is a crucial process in regulated industries, that helps ensure the safety, effectiveness, and high quality of pharmaceutical products and medical devices. Compliance auditing involves a systematic approach that evaluates whether a firm adheres to regulatory requirements, industry standards, and has appropriate internal policies and procedures.
The importance of compliance auditing cannot be overstated, it plays a vital role in protecting public health and safety.
Quality departments may find it difficult to balance the demands of their daily compliance requirements and the requirements of Health Authority regulations.
To ensure ongoing compliance and minimize risk, many organizations are turning to outsourcing GxP compliance audits.
The Advantages of Outsourcing GxP Compliance Audits
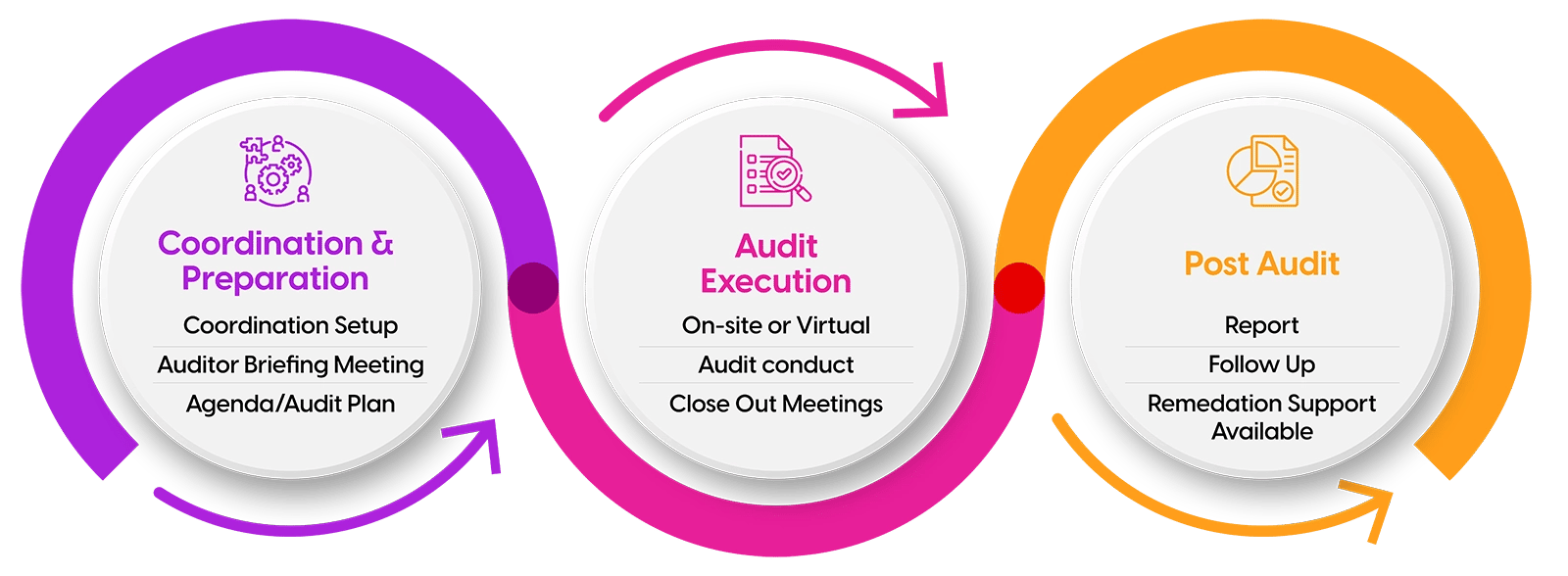
ProPharma's Global Auditing Team consists of experienced professionals with preclinical, clinical, and commercial auditing expertise. Our standardized audit process adheres to respective health authority guidelines worldwide. Freeing up valuable time from our client's quality units.
Our team custom tailors an organized, cost-efficient inspection and report model that is tailored to the type of GxP audit requested while also reflecting current inspection trends. Our extensive experience with CROs, Sponsors, Site Management, IRBs, manufacturers, testing labs, packagers/labelers, distributers, software vendors, and general service vendors is combined with our deep experience in GxP oversight, making ProPharma an ideal partner for complete audit program management, while allowing your quality team to focus on compliance initiatives.
ProPharma's auditing process may be focused on a specific compliance concern or be comprehensive auditing across the span of clinical trial, drug, or device process. We also provide advisory and hands on remediation services to assist with corrective or preventatives actions throughout the quality lifecycle.
Our compliance team is globally situated and vast, we have direct experience in whichever regulated processes are required, saving our clients money on traveling expenses, intimate understanding of compliance topics for efficiency of review, and able to accommodate niche expertise.
Key Benefits of Outsourcing Your Compliance Audits
1. Establishing Long-Term Partnerships
Audits don't just occur once and never again. Many companies have recurring annual audit requirements, or multiple audits throughout the year that need to be performed. Therefore, it's important to choose an audit vendor you can trust and build a relationship with over time. If you are searching for an audit vendor, be sure to understand their level of experience with audits is like the audits you need and ask them about their repeating clients. Do clients partner with them on an ongoing basis? If not, that might be a red flag.
By identifying a trusted audit vendor that has formed hundreds, or even thousands of audits, they should be able to provide a level of transparency and honesty that they have gained from their clients and be able to build a trusted relationship with you. For companies with robust audit needs, your audit vendor should become an extension of your team, helping you solve problems and driving continuous improvements to your program.
2. Audit Coordination Support
What kind of support will you receive from your audit vendor when coordinating and scheduling your audits? Do they have a dedicated team? For example, our clients are assigned a direct point of contact in our Audit Coordinator team, who serve to oversee client expectations, consultant resource alignment and deliverable timeline compliance. Our audit coordinators provide a level of personal touch and flexibility for our clients, helping to ensure the client voice is heard.
3. Access to Expertise
Outsourcing GxP compliance audits can provide access to experienced auditors who specialize in the area regulatory compliance you need. These experts are well-versed in the intricacies of GxP and other regulatory frameworks, and they stay current with the latest changes and trends in the industry. By partnering with such a depth and breadth of professional expertise, organizations can tap into their wealth of knowledge and experience, ensuring a thorough and comprehensive audit.
4. Objective Assessment
An external auditor brings objectivity to the compliance audit process. Unlike in-house personnel who may have a vested interest in the outcome, independent auditors provide an unbiased assessment of a company's operations. This objectivity helps identify potential compliance issues more effectively, leading to more accurate audit results and actionable recommendations for improvement.
5. Cost Efficiency
Maintaining a globally spaced, in-house team of auditors can be a costly endeavor. Organizations must cover salaries, benefits, training, and ongoing professional development for their audit team. By outsourcing compliance audits, companies can significantly reduce these expenses. They only pay for auditing services when needed, avoiding the ongoing overhead costs associated with an in-house team and save travel expenses by utilizing geographically close resources.
6. Focus on Core Competencies
Outsourcing GxP compliance audits allows organizations to focus on their core competencies. Instead of diverting valuable internal resources toward auditing activities, companies can allocate their time and energy to research, development, and other mission-critical tasks. This streamlined approach enhances productivity and accelerates time-to-market for new products.
7. Scalability and Flexibility
The pharmaceutical and biotechnology industries often experience fluctuations in workload due to product development cycles and regulatory changes. Outsourcing compliance audits offers scalability and flexibility. Companies can easily adjust the frequency and scope of audits to align with their specific needs, ensuring they maintain compliance without overburdening their internal teams during peak periods.
8. Risk Mitigation
External auditors bring a fresh perspective and a wealth of experience to the compliance audit process. Their thorough assessments can help identify potential compliance risks and vulnerabilities before they escalate into serious issues. By addressing these concerns proactively, organizations can reduce the likelihood of regulatory penalties and product recalls, protecting their reputation and bottom line.
9. Global Reach
For companies operating on a global scale, outsourcing compliance audits provides the advantage of auditors who are well-versed in international regulations. These auditors can help ensure that facilities and operations in various countries meet the necessary compliance standards, simplifying the complexity of international regulatory compliance.
In Summary
Outsourcing GxP compliance audits offers numerous advantages for pharmaceutical, biotechnology, and healthcare organizations. It provides access to expertise, objectivity, cost efficiency, and scalability while allowing companies to maintain a sharp focus on their core competencies. Additionally, outsourcing helps mitigate compliance risks, protects a company's reputation, and ensures international regulatory compliance. As the regulatory landscape continues to evolve, outsourcing GxP compliance audits remains a valuable strategy for organizations seeking to thrive in these highly regulated industries.
Partner with ProPharma's Global Audit Program
From an individual audit or supporting your full Vendor Management program, onsite or remote, as your auditing partner we are collaborative, flexible, and dynamic. We maintain alignment with existing and evolving industry trends, regulations, and standards across the landscape of GxP auditing, that is all audit compliance types across all regulatory lifecycle stages. Our auditors have significant and direct experience conducting a full spectrum of audit and assessment types, as well as supporting health authority inspections and preparing companies for pre-approval.
ProPharma's audit coordination team provides a direct point of contact to our clients and assists with the facilitation and management of audit logistics, provides periodic progress status updates, maintain audit timelines, and ensure completion of deliverables between client's and auditors.
ProPharma's Compliance Consultant subject matter experts overlay the Global Audit Program with compliance expertise, providing technical support for audit process, and peer review of deliverables. Subject matter expert consultation with clients is also useful when serving as an extension of their clinical and commercial Quality teams, partnering in a constructive and collaborative manner, to evaluate internal and vendor systems for quality improvements.
Also, read our blog post: "Understanding GxP Compliance in Drug and Medical Device Development Lifecycle."
Contact us to discuss ways we can partner with and assist you…Just let us know “when, what, and how” you would like your audit!