Roadmap for Successful IVDR Transition, Part III: Project Management
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching in May 2022. In this blog series, we discuss the final months before the IVDR date of application, how to...
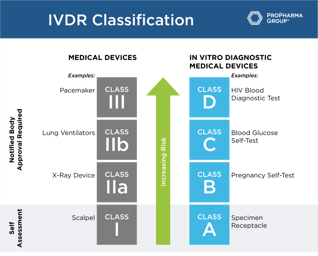
What the IVDR Is and How to Prepare
In May 2022, the IVDD will be repealed by the European Committee, thereby ending the transition period. To ensure you're compliant with IVDR by that date, learn everything you need to know about the...
Roadmap for Successful IVDR Transition, Part II: Technical Documentation & Software
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching (May 2022). In this blog series, we discuss the final months before the IVDR date of application along with...