
Quality & Compliance
Navigating EU GMP Compliance: A Consultant's Guide to Smooth Sailing
Hello, fellow pharma enthusiasts! In the fast-paced world of pharmaceuticals, ensuring Good Manufacturing Practice (GMP) compliance is essential to maintaining product quality and safety. The...

Quality & Compliance
Staying GMP Compliant: A Consultant's Guide to Compliance Bliss
Hello, dear readers and fellow compliance enthusiasts! Welcome to our journey through the labyrinth of Good Manufacturing Practices (GMP) compliance. As a consulting company that provides audit...

7 Critical Factors for Successful Selection of CDMO for Cell and Gene Therapy Manufacturing
Developing, optimizing, and manufacturing Advanced Therapy Medicinal Products (ATMP's), such as Cell and Gene Therapy (CAGT) products is extremely complex. The choice of a reliable Contract...
Prepare for Your Next Audit: A 5-Point GMP Checklist
Ensuring you have full control over your processes, facility, and quality management system (QMS), and ultimately your final product quality, is a demanding and important task. An inability to do so...

Why the FDA Should Never Be Your First Inspection
You can expect several FDA audits throughout your drug development program The Agency’s goal is to protect the public from unsafe products, and one of the best ways to accomplish that goal is by...
Regulatory Sciences
Outsourcing Functions Doesn't Mean You've Outsourced Compliance Obligations
Over the past 20 years, the traditional approach to drug development has expanded to include the outsourcing of a range of testing and manufacturing functions. As a part of their long-term strategic...
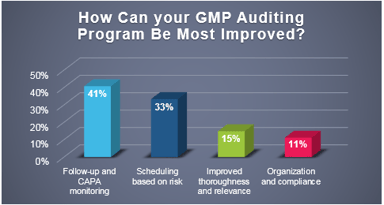
Industry Poll: How Can Your GMP Auditing Program Be Most Improved
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...
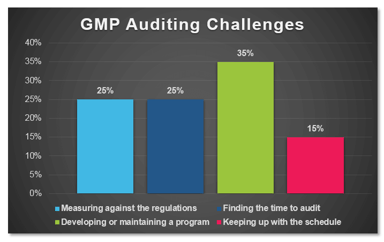
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
Clinical Quality Systems & the Outsourced Model
The landscape of clinical trials is evolving. The changes that are happening are due to the increased number of FDA-regulated trials, as well as a rise in the complexity of clinical protocols. As...
#7: Quality Systems Approach to Pharmaceutical CGMP Regulations
In August 2002, the FDA announced the Pharmaceutical CGMPs for the 21st Century Initiative, which explained FDA’s intent of integrating quality systems and risk management approaches, and had a goal...