
Regulatory Sciences
Regulatory Chemistry, Manufacturing, and Controls (CMC): What to Expect During Drug Development
The key to successful drug development in the US is directional and focused navigation of FDA’s Investigational New Drug (IND) process. The Chemistry, Manufacturing, and Controls (CMC) section is a...
Regulatory Sciences
FDA Animal Rule: Overview & Impact on Drug Development
What is the Animal Rule? The Animal Rule, a regulation set by the US Food and Drug Administration (FDA), applies to the development and testing of drugs and biological products intended to reduce or...

Regulatory Sciences
The Impact of a US Government Shutdown on the Food and Drug Administration
This article was originally published in September 2023, and has been updated to reflect an upcoming potential government shutdown. As U.S. lawmakers return from the President’s Day holiday, they are...

Demystifying CAPA Management: Overcoming Challenges in the Fast-Paced World of GMP
This article has been updated since its original publication date. Navigating the complexities of Corrective Action / Preventive Action (CAPA) in the drug and medical device industries often poses a...

Illuminating FDA's 2023 BLA Approvals: A Comparative Analysis
The FDA's Biologics License Application (BLA) approvals in 2023 have marked a significant chapter in medical innovation, embodying precision and transformative therapies. A closer examination of the...

2023: A New Chapter in FDA Drug Approvals - A Resurgence of Innovation
The year 2023 unveiled an eventful chapter in FDA drug approvals, heralding a resurgence of innovation after a brief decline. This period represented not just a numerical rebound but a meaningful...

FDA’s Updated Software Guidance
The FDA has recently published a number of software related guidance documents, covering topics such as off-the-shelf software, cybersecurity, closed-loop-control, and predetermined change control...

Navigating FDA User Fee Updates for Fiscal Year 2023
As the U.S. Government begins its fiscal year on October 1, it signifies the annual revisions in FDA User Fees, which have an impact on applications and facilities associated with Prescription Drugs...

FDA Proposes New, Easy-to-Read Medication Guide for Patients
The US Food and Drug Administration (FDA) has proposed a new, easy-to-read medication guide for patients known as the Patient Medication Information (PMI)1. The new medical guide will be required for...

FDA's Concern over Diethylene Glycol (DEG) and Ethylene Glycol (EG) Contamination
Recent contamination of several drug products in India resulting in fatalities have prompted a new FDA guidance entitled "Testing of Glycerin, Propylene Glycol, Maltitol Solution, Hydrogenated Starch...

Quality & Compliance
The Importance of Responding to FDA 483 Observations
This article has been updated since its original publication date. The FDA has an established policy that allows companies 15 days to respond in writing to the FDA after issuance of a 483...

Quality & Compliance
FDA Form 483: Common Pitfalls You Can Avoid
This article has been updated since its original publication date. FDA Form 483 requires a written response in which you must make it clear that you are taking the observations, and your...

Quality & Compliance
MoCRA 2022: Updated Requirements for Cosmetic Companies
MoCRA enacts the most significant expansion of the US FDA to regulate cosmetics since 1938. After 85 years of effectiveness the Modernization of Cosmetics Regulation Act of 2022 or "MoCRA" was...
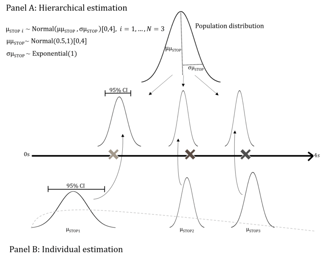
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....