Clinical Research Solutions
FDA Form 483: Common Pitfalls You Can Avoid
This article has been updated since its original publication date. FDA Form 483 requires a written response in which you must make it clear that you are taking the observations, and your...

Clinical Research Solutions
MoCRA 2022: Updated Requirements for Cosmetic Companies
MoCRA enacts the most significant expansion of the US FDA to regulate cosmetics since 1938. After 85 years of effectiveness the Modernization of Cosmetics Regulation Act of 2022 or "MoCRA" was...
Clinical Research Solutions
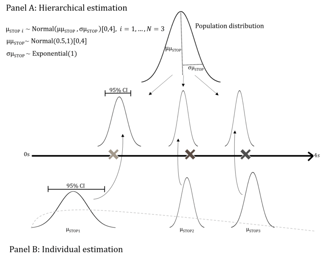
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

Clinical Research Solutions
FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
Clinical Research Solutions
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
Clinical Research Solutions
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...

Clinical Research Solutions
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA) , FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...

Clinical Research Solutions
From Idea to Market: The Five Stages of Product Development
The exact product development process for medical devices differs from region to region, with different regulatory expectations that need to be met in the EU, USA, UK and other regions. These precise...
Clinical Research Solutions
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement
Pharmaceutical companies should understand EU Health Technology Assessment (HTA) authorities requirements early in the product development phase. Engagement with HTA authorities during clinical...