
Medical Information
Navigating EMA and Global Regulations for Medical Information Services
Medical Information services are essential to ensure healthcare professionals (HCPs), patients, and regulatory bodies receive accurate, timely, and compliant information. From managing inquiries to...

Regulatory Sciences
Advancing Digital Transformation in Medicines: EMA's Successful ePI Pilot
The European Medicines Agency (EMA) and the Heads of Medicines Agencies (HMA) have recently concluded a successful pilot project on electronic product information (ePI), marking a significant...

Regulatory Sciences
Navigating the New EU Health Technology Assessment Regulation (HTAR): Are You Ready?
The HTAR in the EU has come into force – what Health Technology Developers (HTDs) need to know Getting your health technology product to the market is complex and usually consists of several steps...

Regulatory Sciences
Leveraging Single-Arm Trials for Regulatory Approval: Insights from EMA's Reflection Paper
The pharmaceutical industry has shown a growing interest in single-arm trials due to their potential to expedite drug development. However, several challenges and concerns remain. The European...

Regulatory Sciences
How to Create Successful Pricing and Reimbursement of your Pharmaceutical Product in the Nordics
Our Nordic region features world-class healthcare. Understanding the Nordic healthcare and market access landscape is crucial for successful pricing and reimbursement of your pharmaceutical product....

Pharmacovigilance
Enhancing Animal Health Compliance with Comprehensive Medical Information (MI) and Pharmacovigilance (PV) Services
Comprehensive Animal Health Medical Information (MI) and Pharmacovigilance (PV) support services in the veterinary industry are a must-have for companies who want to ensure the safety, efficacy, and...

Regulatory Sciences
UK/EU Regulatory Developments Review 2023
Introduction The year 2023 has been remarkable in terms of regulatory developments within the UK and EU, characterised by substantial changes, innovative approvals, and strategic initiatives in the...

Regulatory Sciences
Navigating Expedited Regulatory Pathways in Europe: The Latest Insights and Advancements
Are you truly up to speed on the most effective strategies to expedite the approval process for your innovative product in Europe's evolving regulatory landscape? In the United States (US), the Food...

EMA Policy 0070: Back on the Road to Transparency
By now, most involved in the clinical trials disclosures field are aware of the upcoming reinstatement of the European Medicine Agency's (EMA) landmark transparency regulation: Policy 0070. Policy...

Quality & Compliance
Integrity and Reliability Concerns in Bioequivalence Studies: An Insight into the Synapse Labs Inspection
In a globalized pharmaceutical industry, ensuring the integrity and reliability of clinical data is of utmost importance. Recently, the Spanish Medicines Agency conducted a comprehensive Good...

EMA’s Regulatory Science Strategy to 2025 is on Track
Key messages from the mid-point achievements report To strengthen regulatory and scientific support for innovative medicines and diagnostics development, in March 2020 EMA published its Regulatory...
Regulatory Sciences
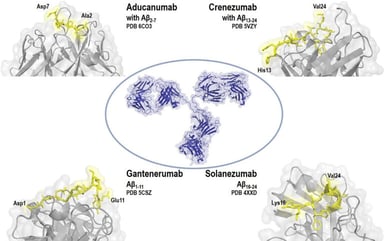
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Regulatory Sciences
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin). The vaccine is only authorised for...
Regulatory Sciences
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement
Pharmaceutical companies should understand EU Health Technology Assessment (HTA) authorities requirements early in the product development phase. Engagement with HTA authorities during clinical...

Regulatory Sciences
UK Paediatric Investigational Plans – what do you need to know?? …and how is it all working in practice??
If a marketing authorisation is planned to be submitted in England, Scotland, and Wales (GB), an MHRA-approved paediatric investigational plan (PIP) is required. Up until January 1, 2021, PIPs were...
