Quality Agreements and the Pharmaceutical Supply Chain
Over the past several years, outsourcing within the pharmaceutical supply chain has become common. The rise of the “virtual” pharmaceutical company has resulted in a complex supply chain, with the...
FDA Clarifies its Definition of "Convenience Kit"
On January 4, 2016, the FDA published a draft guidance entitled, “Unique Device Identification: Convenience Kits.” The draft guidance comes after the Agency’s 2013 final rule that established a...
Obtaining IND-Related Feedback from FDA
What to do if you experience delays in obtaining IND-related feedback from the FDA: In December 2015, the FDA released a draft guidance, which provides IND sponsors with a number of recommendations...
Regulatory Sciences
Formal Meetings with the FDA Regarding Biosimilars: What’s Changed?
Recently, biosimilars have made a definite appearance on the FDA’s radar, an expected result after the first biosimilar product gained FDA approval in March and the FDA’s release of four final...
There’s an App for That: FDA Launches Orange Book Express App
On November 9, 2015, FDA launched the Orange Book Express application, which provides a list of the drug products approved by the FDA. What is the Orange Book? The Drug Price Competition and Patent...
FDA Investigates Risk of Off-Label Tramadol Use in Children
FDA is investigating the use of tramadol, a narcotic-like pain reliever, in children aged 17 years and younger after learning of the “rare but serious” risk of slowed or difficult breathing....
FDA Updates Informed Consent Guidance
FDA recently updated its informed consent guidance in the form of an Information Sheet. The new document reflects the Agency’s current thinking on the informed consent process utilized in...
Clinical Research Solutions
What Can Testosterone Gels Teach Us About Abuse-Deterrent Opioids?
For most drugs, the process of developing and obtaining FDA approval of a generic version is simple and well-defined: sponsors must only prove that their product is pharmaceutically equivalent and...
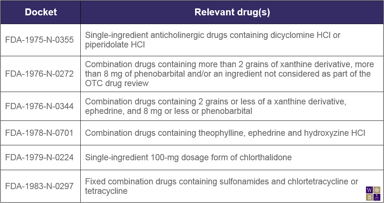
Unapproved Codeine Products and Some DESI Drugs need FDA Approval or Cease Marketing
In a Federal Register notice published today, the Food and Drug Administration (FDA) announced its intention to take enforcement action against misbranded or unapproved prescription products...
Clinical Research Solutions
FDA Proposes Controversial Rule Allowing ANDA Applicants to Change Drug Labels
On Nov. 13, 2013, the Food and Drug Administration (FDA) published a Proposed Rule in the Federal Register. The title of the Proposed Rule is “Supplemental Applications Proposing Labeling Changes for...
Waivers for Carcinogenicity Studies? Not So Fast!
The recently published request for comments regarding the Proposed Change to Rodent Carcinogenicity Testing of Pharmaceuticals signals intent by regulatory agencies to alter what Sponsors consider in...

Regulatory Sciences
FDA Releases New Guidance on Meetings with Sponsors of Biosimilars
On March 29, 2013, the FDA made available a draft guidance for Sponsors of biosimilar products outlining the procedures and processes for meetings with the FDA. Although much of guidance for the...
Cetero FDA Action
If your company has used Cetero Research's Houston facility to conduct bioanalytical studies between April 1, 2005 and June 15, 2010, your marketing applications may need to be repeated or confirmed....
