October 26, 2020

October 26, 2020

The Annual Product Review (APR), also known as the Annual Product Quality Review (APQR), is required for marketed products in an FDA-regulated environment. You may ask, “Why would I want to perform an APR?”. The Annual Product Review creates an opportunity to analyze your product’s characteristics, trends, and issues to identify potential process and product improvements.
The purpose of the APR is to generate, review, and provide approval of the report for all products manufactured at the firm where such reviews are required by the regulatory authorities in the intended product markets.
Specifically, the objective of the APR is to provide an evaluation of data and trends to:
The key word mentioned above is opportunity. As an unbiased, retrospective evaluation, the Annual Product Review presents an opportunity to create efficiencies and demonstrate that the product remains in a state of control.
Now, you may also ask, “Do I have to perform an APR?”. The short answer is yes. Why? Primarily, it is required by law, by the FDA’s Code of Federal Regulations (CFR).
Now that you know you must write the APR; you may be wondering how to get started. What are the primary components that make up the APR?
The key components of the APR include:
How about best practices? A few things to review and consider:
It sounds like a big task, and it is. To fully understand the APR process, look at your firm’s unique procedure on Annual Product Reviews, and be sure you understand the FDA's CFR requirements.
The Annual Product Review is a fantastic opportunity to learn about your product and its characteristics and identify opportunities for improvement. While it may not be a riveting, thriller-style read, I recommend a read-through of your product’s latest APR report. And, if you’re lucky enough to be part of writing an APR, I say, “congratulations”.
If you need help with your Annual Product Review(s), ProPharma Group is here to assist. We can:
Remember: The Annual Product Review is not only a great opportunity for improvement, they are required by law. If you have questions or concerns regarding your current APR practices, our compliance experts are here to assist. Contact us today!
TAGS: Life Science Consulting
November 13, 2023
The FDA's Breakthrough Devices Program is intended to expedite the development of innovative technologies for patients with life-threatening or irreversibly debilitating diseases or conditions. The...
October 25, 2022
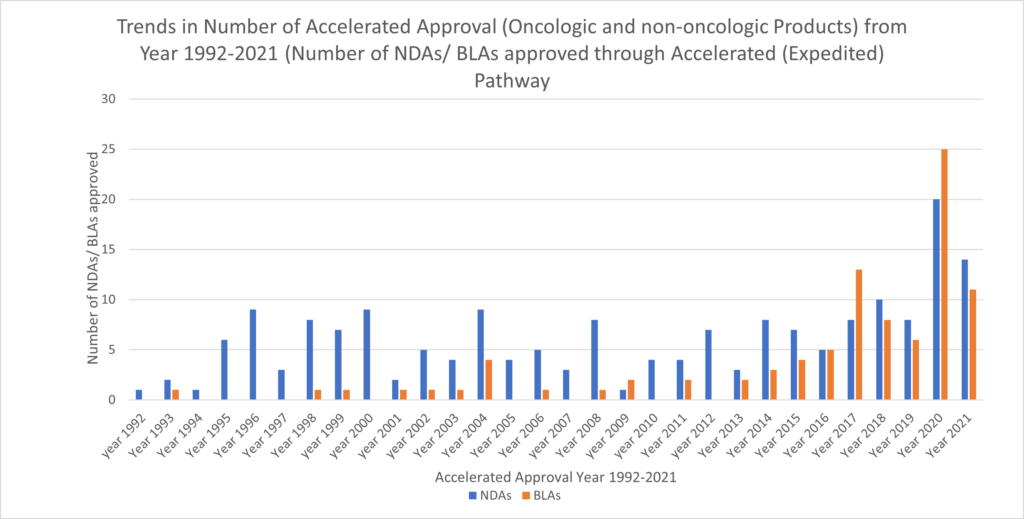
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...