Regulatory Sciences
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
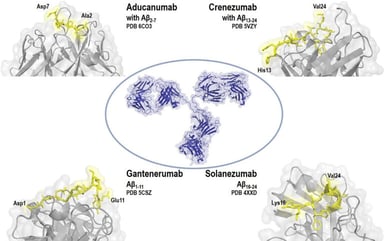
Orphan Drug Designations in the US and EU
What is an Orphan Drug Designation? The Orphan Drug Designation (ODD) program in both the United States (U.S.) and European Union (E.U.) qualifies sponsors to receive potential incentives to develop...
Regulatory Sciences
Competition Between Orphan Drug Sponsors – Good for Patients, Strategic Complexities for Sponsors
The regulations developed by the FDA for implementing the Orphan Drug Act contain a number of features that are intended to maximize benefits to the orphan disease patient population, but which pose...