
Regulatory Sciences
ANDA Development Made Clear: Expert Advice on Submission Strategy, Labeling, and Patent Considerations
Inside our Q&A session with former FDA labeling reviewer, Marshall Florence, PharmD., regarding ANDA submission strategy, labeling, and how to effectively use the OrangeBook as a strategic resource....

Regulatory Sciences
Leveraging Online FDA Information to Accelerate ANDA Timelines
In the race to generic drug approval, timing is everything. Delays in Abbreviated New Drug Application (ANDA) submissions or setbacks during FDA review can mean missed market opportunities,...

Regulatory Sciences
FDA Drug Labeling Requirements & Regulations: What’s in Your Label?
Why Does Pharmaceutical Product Labeling Matter? Do you know what’s in your product’s labeling and what it is saying about your product? More importantly, do you know why that matters? A drug’s label...
Regulatory Sciences
Pediatric Labeling Best Practices
A large percentage of drugs are used off-label in pediatric patients. Unfortunately, when a patient uses a drug off-label, the drug is being used without FDA approval, which might result in...
Regulatory Sciences
6 Compliance Tips to get FDA Approval for Your Pharmaceutical Project
FDA Approval Process Overview The Food and Drug Administration (FDA), as part of the United States (US) Department of Health and Human Services, is the regulatory agency responsible for the review,...
Regulatory Sciences
#2: Labeling for Human Prescription Drug and Biological Products – Implementing the PLR Content and Format Requirements
In 2013, the FDA released a guidance entitled "Labeling for Human Prescription Drug and Biological Products – Implementing the PLR Content and Format Requirements." This guidance was finalized after...
Regulatory Sciences
The FDA’s Enforcement of Section 503B of the Federal Food, Drug, and Cosmetic Act
The FDA publishes weekly enforcement reports highlighting drugs that have been recalled during the previous week. Over the past several months these reports have been littered with hundreds of...
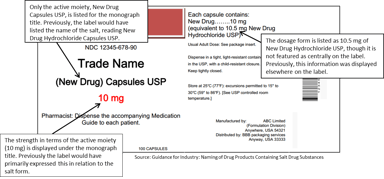
Regulatory Sciences
FDA Guidance Follows USP Salt Policy & Aims to Make Conversions Between Salt Forms Simpler
The Food and Drug Administration (FDA) recently published a draft guidance that outlines the “Naming of Drug Products Containing Salt Drug Substances” in accordance with the recently implemented USP...
Regulatory Sciences
FDA Comments on Proposed Prescription Drug Labeling
Standardized prescription drug labeling was implemented by FDA in 1979. In the following years as labeling became more complex, FDA re-evaluated its usefulness and published a final rule in 2006...
Regulatory Sciences
Label Changes Related to the QTc Interval
In a recent FDA Drug Safety Communication, the Agency announced that the Celexa (citalopram hydrobromide) label now has revised dosing recommendations based on evaluations of post-marketing reports...