Regulatory Sciences
The Unique Challenges of Gaining Approval for Drug-Device Combos
A combination product is composed of any combination of a drug and a device; a biological product and a device, a drug and a biological product, or a drug, device, and a biological product. Consider...
3 Factors to Consider in the Manufacturing Phase for Drug Device Combination Products
Combination products represent an important and growing category of therapeutic and diagnostic products. They come in several configurations and can be composed of any combination of a drug and a...
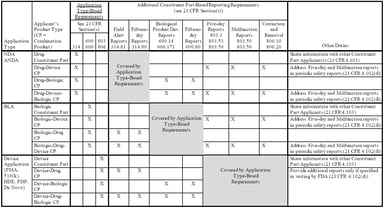
Pharmacovigilance
Understanding the New Combination Product PMSR Guidance Documents and Impact on Industry
On March 20, 2018, the US Food and Drug Administration (FDA) released two new guidance documents to help companies comply with the December 20, 2016 final rule establishing postmarketing safety...