
Regulatory Sciences
Highlights from FDA's Analytical Test Method Validation Guidance
Recently, the FDA updated a long-standing, decades old guidance on analytical test method validation based on revisions of the ICH Q2(R2) guidelines. Traditional test method validation requirements...
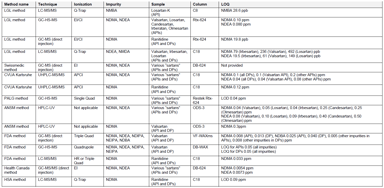
Analytical Methods for the Investigation of Carcinogenic Nitrosamines in APIs and Drug Products
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...
Analytical Method Transfer Checklist
As you plan for an upcoming Tech Transfer, have you considered if you are appropriately prepared to conduct an analytical method transfer? With the simple analytical method transfer checklist...
Quality & Compliance
Understanding Analytical Method Validation
The core of my background was built upon analytical development science for biotechnology companies including Celera and Genentech. It became routine to develop analytical assays to test products...